Answer
387k+ views
Hint: We know that the conductometric titration may be a laboratory method of quantitative chemical analysis wont to identify the concentration of a given analyte during a mixture. Conductometric titration involves the frequent adding of a reactant to a reaction mixture and consequently the records of the resultant change within the electrolytic conductivity of the reaction mixture
Complete step by step answer:
First we see the principle of the conductometric titration.
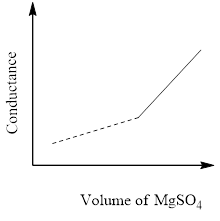
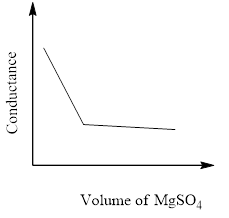
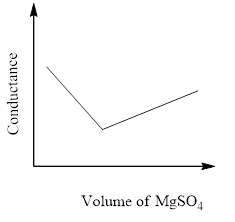
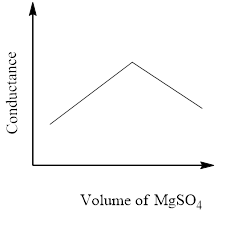
The principle of the conductometric titration is in a titration process, one ion is replaced with another and therefore the variation within the ionic conductivities of those ions directly impacts on the common electrolytic conductivity of the solution.
The conductivity that's measured in an electrolyte solution depends on the sort and concentration of the ions. As long since the reaction is taking its course the conductivity drops when the quality solution is in excess, the conductivity rises again.
So, the correct answer is Option C.
Note:
Now we can discuss about the advantages and drawbacks of conductometric titration as,
Advantages Conductometric Titration:
This process is extremely useful within the titrations of very dilute solutions and weak acids.
The end-point of this method of titration is extremely sharp and accurate in comparison to a couple of other titration processes.
This type of titration is applicable for solutions that are colored or turbid, and that the endpoint of the titration with normal indicators can't be observed easily by the human eye.
Conductometric titration has numerous applications in acid-base titrations, redox titrations, precipitation titrations, and sophisticated titrations.
The major drawbacks of this sort of titration include:
Only a couple of specific redox titrations are often through with the assistance of this process. This is often because the conductivity of the answer is masked by relatively high hydronium ion concentration.
Complete step by step answer:
First we see the principle of the conductometric titration.
The principle of the conductometric titration is in a titration process, one ion is replaced with another and therefore the variation within the ionic conductivities of those ions directly impacts on the common electrolytic conductivity of the solution.
The conductivity that's measured in an electrolyte solution depends on the sort and concentration of the ions. As long since the reaction is taking its course the conductivity drops when the quality solution is in excess, the conductivity rises again.
So, the correct answer is Option C.
Note:
Now we can discuss about the advantages and drawbacks of conductometric titration as,
Advantages Conductometric Titration:
This process is extremely useful within the titrations of very dilute solutions and weak acids.
The end-point of this method of titration is extremely sharp and accurate in comparison to a couple of other titration processes.
This type of titration is applicable for solutions that are colored or turbid, and that the endpoint of the titration with normal indicators can't be observed easily by the human eye.
Conductometric titration has numerous applications in acid-base titrations, redox titrations, precipitation titrations, and sophisticated titrations.
The major drawbacks of this sort of titration include:
Only a couple of specific redox titrations are often through with the assistance of this process. This is often because the conductivity of the answer is masked by relatively high hydronium ion concentration.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Three beakers labelled as A B and C each containing 25 mL of water were taken A small amount of NaOH anhydrous CuSO4 and NaCl were added to the beakers A B and C respectively It was observed that there was an increase in the temperature of the solutions contained in beakers A and B whereas in case of beaker C the temperature of the solution falls Which one of the following statements isarecorrect i In beakers A and B exothermic process has occurred ii In beakers A and B endothermic process has occurred iii In beaker C exothermic process has occurred iv In beaker C endothermic process has occurred

What is the stopping potential when the metal with class 12 physics JEE_Main

The momentum of a photon is 2 times 10 16gm cmsec Its class 12 physics JEE_Main

How do you arrange NH4 + BF3 H2O C2H2 in increasing class 11 chemistry CBSE

Is H mCT and q mCT the same thing If so which is more class 11 chemistry CBSE

Trending doubts
Difference Between Plant Cell and Animal Cell

Difference between Prokaryotic cell and Eukaryotic class 11 biology CBSE

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Fill the blanks with proper collective nouns 1 A of class 10 english CBSE

Change the following sentences into negative and interrogative class 10 english CBSE

Give 10 examples for herbs , shrubs , climbers , creepers

Name 10 Living and Non living things class 9 biology CBSE

What organs are located on the left side of your body class 11 biology CBSE

Who gave the slogan Inquillab Zindabad A Bhagat Singh class 10 social science CBSE