In a series of rooms in the Fly Facility of the Department of Genetics at Cambridge University, around 5m fruit flies are kept in test tubes at any given time. They’re stored at different temperatures to determine varying lengths of life cycle – at 25C, it’s about 10 days; at cooler temperatures as long as five weeks.
Out in the wild, there is no pest quite so sympathetic to human needs as the humble fruit fly. It may have spent the summer feasting on the contents of your fruit bowl, but not until your assembled plums and peaches were starting to rot. But while gastronomic sensitivity is a characteristic to be applauded in a fly, the drosophila, to give it its official title, has more going for it than good table manners.
For the past century, it has also performed the vital function of a scientific and medical research tool. Today, it’s often the first stop in research into a wide range of human illnesses, including Alzheimer’s disease, Huntington’s disease, spastic paraplegia, cancer and obesity. By comparison with mice and dogs, let alone apes and humans, it’s massively cheap and easy to work with and there is little chance of drawing protest from even the most radical anti-vivisectionist.
In many respects its position as a vital research tool is a historical accident. Between 1910 and 1915, the pioneering American geneticist Thomas Hunt Morgan worked on Drosophila melanogaster in his renowned Fly Room at Columbia University and showed that genes provide the basis for chromosomal heredity, for which he won a Nobel prize. It was a critical breakthrough, but there was no particular reason that it had to be made via the fruit fly. Yet ever since then, the tiny drosophila has been at the forefront of genetic research. In the 1920s, another American geneticist, Hermann J Muller, demonstrated that radiation leads to genetic mutation in fruit flies. The reason we’re careful about exposure to x-rays is no small part due to Muller’s work.
But some of the mutations that Muller produced, such as flies with legs coming out of their heads, later played into the postwar period of atomic paranoia, informed George Langelaan’s short story The Fly, which was made into a film first in the 50s and then remade by David Cronenberg in the 1980s.

In the story and the films, a scientist mutates into a fly, an idea that’s disturbing precisely because we see ourselves as being so utterly different from flies, with their strange bodies and massive, honeycombed heads and distinctly creepy habits. This deep-seated anxiety about flies has led to some famous misunderstandings of biology. The most egregious example was in 2008, when Sarah Palin, running for vice-president in the US presidential elections, told an audience that their money was going “to projects that have little or nothing to do with the public good. Things like fruit fly research in Paris, France. I kid you not.”
Despite Palin’s clueless misgivings, one of the reasons that flies have become important to much genetic and medical research in relation to humans is that they bear a remarkable genetic similarity to us. The sci-fi fear of a fly’s otherness may well be based, somewhere way down, on its unsettling closeness to us.
It was Michael Ashburner, the godfather of fruit fly research at Cambridge, who first established that of the genes that in their mutant form cause diseases in humans – cystic fibrosis being one example – around 75% have very similar equivalents in fruit flies. When Ashburner started out in the 1970s, flies were kept in milk bottles in a temporary lab on the outskirts of Cambridge. As a result of his foundational work, which includes his classic book Won For All: How the Drosophila Genome Was Sequenced, Cambridge has become arguably the world’s leading centre of fruit fly research.
Its Fly Facility is run by one of Ashburner’s former PhD students, Simon Collier, who showed me around the labs and fly storage of the facility. He’s been working with fruit flies for 25 years and in that time he’s come to know and appreciate many of their hidden – to most human observers – characteristics.
“If you take a tube of flies and leave it here you notice that they have behaviours,” he says. “The males court the females. They follow the females and put one wing out and it vibrates. There’s a guy in Leicester who’s researched this and what they’re doing is producing a kind of love song.”
Apparently, the females are not looking for a long-term relationship and, indeed, they’re likely in their short life span to have multiple partners. The problem with this for geneticists is that they store sperm so paternity is a disputed issue. To counter the confusion of this flagrant promiscuity, geneticists tend to work with virgin females.
How can they tell? I ask.
“The look in their eye,” Collier says drily.
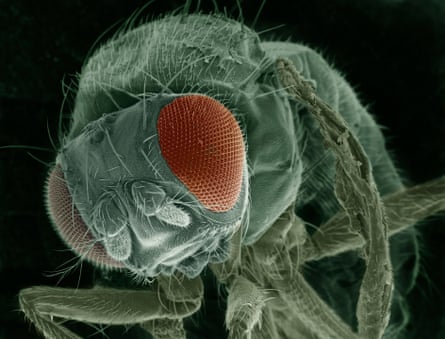
He shows me a magnification of a load of fruit flies that have been knocked out by carbon dioxide. They’re still twitching but essentially stationary. He points out the differences between males (a bit smaller) and females and shows that young flies – virgins if you like – are pale and unpigmented.
He explains that for research purposes special chromosomes have been developed that enable geneticists to trace exactly what genes have been inherited. With mice, for example, it’s necessary to check gene by gene what’s been inherited, which makes genetic research much more time-consuming and costly.
Because the fruit fly’s life cycle is so short and they reproduce so fast (sexually maturity is reached within eight days of hatching), drosophila are ideal subjects for the study of inherited traits, including genetic abnormalities, over many generations.
Still, to the layman, even one slightly more versed in the exigencies of scientific research than Sarah Palin, there’s something profoundly counterintuitive about doing genetic research on flies. For one thing, they’re so small. Doesn’t that make it a whole lot trickier?
Collier shakes his head. “It’s pretty straightforward if you want to look at the fruit fly’s genome. You just put them in a tube and squish them up and do some simple DNA extraction. What’s more complex is going the other way – implanting genetic material into them.”
He takes me to a special lab where this procedure is carried out. They take the fly larvae and strip the eggs off their shells by sticking them in bleach. Then with a long and extremely fine needle, the relevant genetic material is injected into the posterior of the eggs where the germline cells are located.

Given that an egg is about 0.5mm in length (about the size of a grain of sand), and the DNA injected into it is about the volume of a millionth of a drop of water, you can see how delicate an operation it is. It takes about six months to master the minute motor skills necessary to do the job.
“Usually half the embryos will survive that procedure,” says Collier. “And we can breed from them and study them.”
But study what exactly? And to what end?
Collier introduces me to two colleagues who are active in fruit fly research, a reader in genetics called Cahir O’Kane and Damian Crowther, a director of neuroscience research and development at the pharmaceutical giant AstraZeneca, who still retains links to the Fly Facility.
We go out to lunch and talk fruit flies. I ask Crowther first of all why he left academic research to go into industry.
“The story I tell,” he says, “is friends, and perhaps even enemies, would always introduce me as, ‘This is Damian, he’s the fly guy’. In my time, I’ve been a registrar in neurology and a research scientist in many areas – I didn’t think fly guy really summed me up.”
O’Kane echoes the point and both men agree that fly research has not brought them either the professional or social recognition they believe their work warrants.
What seems to matter most, in terms of professional appreciation at least, is what is known as “translation”, that is, turning the findings of fly research into productive contributions to medical applications for humans. There is little doubt that fly research does contribute, in a wider sense of biological understanding of how all organisms work, but also in specific examples of human disease. However, it’s not easy to make direct links.
It’s problematic, for obvious reasons, to go from fly tests to human tests; there usually needs to be a whole range of intervening stages in which other scientists take over and, inevitably, take the spotlight and accreditation.
“I can’t tell you that there’s a drug that I’ve tested on fruit flies [with an artificially created version of Alzheimer’s disease] that’s benefited the fruit fly that’s then gone on to benefit the human,” says Crowther.
But, he explains, Alzheimer’s involves the overproduction of proteins that form plaque in the brain that destroys neurons. “So you can make models of flies that produce these proteins, get their own plaque and die,” he explains, “and you can then test various ways of preventing the plaque formations.”
Fruit flies don’t naturally develop Alzheimer’s, although they have all the genetics of the Alzheimer’s pathway in their brain.
“You have to give them human equivalent genes and push it really hard to get them to have Alzheimer’s in three weeks,” explains Crowther.

Instead of thinking of fly research as a direct route to medical breakthroughs, it’s better to see it, Crowther argues, “as a way of doing quick and dirty research”. He believes that because it’s so cheap, it should be used in a multiplicity of ways that might point the direction to more productive lines of research. And where there are drugs that have already been tested on humans and have passed safety requirements but have failed in their efficacy with the targeted complaint, they could be retargeted by first testing them on flies.
“The thinking around really bad diseases like motor neurone disease, Huntington’s disease, is if you can get anything to work in a cell culture replicated in an organism, that’s the beginning – as long as it’s safe – of quickly getting it to patients,” says Crowther.
O’Kane is particularly suspicious of overblown claims for translation. For him, the beauty of the fruit fly is that it is a organism that rewards study in a larger context.
“I’m interested in it because I think it’s a great system for finding out how living things in general can work. I think by understanding the principles of how flies work then you can make better predictions for humans. The better you understand how we work the more rational you can be about trying to design therapies; I think even without directing your work towards therapy, then you can think more intelligently about therapies five or 10 years down the line.”
It has been said, not least by Collier, that more is known about the biology of the drosophila than any other animal on Earth. For instance, we know that fruit flies have a kind of built-in compass in their brains that enables a sense of direction. As all animals need to know how they move, it’s not unreasonable to assume that it’s a form of universal computation.
Another study has shown that male fruit flies that are rejected by a female mate are more inclined to drown their sorrows in food spiked with alcohol than male fruit flies that had successfully mated. Again, a brain chemical that regulates the flies’ appetite and is predictive of their thirst for alcohol has an equivalent that has been linked to alcohol consumption in humans.
In another study, this time at Oxford, it was found that fruit flies are capable of what might be termed thoughtful deliberation. Rather than making purely impulsive decisions, they take time to react when presented with a difficult choice.
In other words, once again their behaviour could be described as human-like. It seems that the common ingredient in both human and fly behaviour is a gene called FOXP, which is closely linked to cognitive development in humans. Flies with defective FOXP take longer to arrive at a decision, just as defects in the human type of FOXP have been correlated with low intelligence.
It is this long and valuable history of study of the drosophila that should guarantee its continued involvement in genetic research. But much of what has been good about working with flies is now being replicated in human stem cell research, which has the added advantage of being species-relevant. This was the other reason why Crowther moved into the private sector: the competition from stem cells meant that he found it increasingly difficult to get fruit fly-based research funded.
“The fly is yesterday’s guy,” he says, “yesterday’s technology. For me, stem cells are the next fruit fly.”
O’Kane looks quite glum at the prospect and argues that there have been questions over the future of fruit fly research ever since he started his lab 25 years ago. But he maintains that the work he’s done in hereditary spastic paraplegia would have taken 10 times longer to perform with mice. O’Kane has grown to appreciate the rich biological and social development of fruit flies in the time he’s been working with them.
“The more you look at their behaviour,” he says, “the more sophisticated you realise they are. Even in aggression, how a male fruit fly behaves in a fight will depend on his previous experience of fighting with other males and what the other males’ previous experience of fighting is. It’s amazing to think about all the machinery that’s involved to be able to do that. Obviously we’re more sophisticated, because we’re studying them, they’re not studying us.”
For the time being, until some over-ambitious genetics professor does manage to mutate into a fly, that’s the way the relationship should continue. And both humans and fruit flies, it turns out, can drink to that.

Comments (…)
Sign in or create your Guardian account to join the discussion