Abstract
Serious concerns have arisen regarding urbanization processes in western Amazônia, which result in the creation of artificial habitats, promoting the colonization of malaria vectors. We used structural equation modelling to investigate direct and indirect effects of forest cover on larval habitats and anopheline assemblages in different seasons. We found 3474 larvae in the dry season and 6603 in the rainy season, totalling ten species and confirming the presence of malaria vectors across all sites. Forest cover had direct and indirect (through limnological variables) effects on the composition of larval anopheline assemblages in the rainy season. However, during the dry season, forest cover directly affected larval distribution and habitat variables (with no indirect affects). Additionally, artificial larval habitats promote ideal conditions for malaria vectors in Amazonia, mainly during the rainy season, with positive consequences for anopheline assemblages. Therefore, the application of integrated management can be carried out during both seasons. However, we suggest that the dry season is the optimal time because larval habitats are more limited, smaller in volume and more accessible for applying vector control techniques.
Similar content being viewed by others
Introduction
Urbanization, loss of native vegetation and habitat modification have dramatically altered tropical forests globally1,2. These land use changes have led to losses of biodiversity and ecosystem services3,4 and affected the population dynamics of vector mosquitoes with important consequences for public health5,6. Mosquitoes are key vectors of human diseases, globally transmitting more than 17% of all infectious diseases. Dengue and malaria cause 440,000 deaths annually, and the large numbers of people infected often overloads healthcare systems7. In Brazil, >99% of malaria cases occur in Amazônia, including 63,361 cases of malaria in the state of Amazonas in 20198,9.
Serious concerns have been raised about urbanization in western Amazônia10, one of the world’s richest biodiversity regions, which houses more than 7,400,000 humans11. Urbanization and human expansion in the region have been increasing both in urban centers and in peri-urban areas12,13. The relationship between deforestation and malaria dynamics is complex and interconnected. For example, deforestation and urbanization increase the number and distribution of habitats available for the malaria vector Anopheles darlingi Root, 1926, thereby expanding malaria transmission6. These anthropogenic impacts also increase malaria cases resulting from increased contacts between humans and vector species14. In contrast, high numbers of malaria cases reduce deforestation through socio-economic mechanisms15.
Disentangling the role of several mechanisms by which deforestation affects mosquito diversity, distribution and abundance in tropical forests is challenging, because forest losses likely have direct and indirect effects on larvae and adults. For example, forest loss can reduce anophelines in the subgenus Kerteszia, which depend on tree holes and bromeliads for larval habitats16. The loss of native vegetation can also change microclimatic conditions, such as temperature and humidity, which in turn may affect mosquito population dynamics17. Land use changes may also influence mosquito species by reducing their host taxa, such as non-human primates, thereby reducing pathogen transmission18.
Deforestation and urbanization processes often create new artificial larval habitats, such as trash, dams, ponds, and clay pits, promoting colonization by mosquitoes, including malaria vectors in Amazônia19,20. The amount of forest around artificial habitats also influences water quality variables, such as temperature, dissolved oxygen, sediments, and dissolved and suspended organic matter. Seasonality can also modulate water quality21. For example, in the dry season, lentic habitats are reduced, leading to increased primary production and dissolved solids and decreased pH, which are positively correlated with the presence of Anopheles species in aquatic systems22,23. Therefore, distinguishing the direct (mediated only by forest loss) and indirect (forest loss effects on larval habitats) effects of forest loss on mosquito diversity and abundance is fundamental for understanding and predicting mosquito assemblages.
In this study we assessed how forest loss might directly and indirectly (through limnological variables) affect Anopheles assemblages in Manaus, Amazonas. We hypothesized that forest loss and limnological variables would affect mosquito assemblages in the rainy and dry seasons differently because of their differing effects on mosquito habitat conditions (Fig. 1). We expected that the forest cover gradient and limnological variables would have stronger effects on larval assemblages during the dry season. Forests play a critical role in retaining moisture (including larval habitats) and filter some forest dependent species. Also, during the dry season, water levels are decreased and organic matter is concentrated, strengthening effects of limnological variables on mosquito assemblages.
Conceptual structural equation model. The image was created using Abode Illustrator 2020 (https://www.adobe.com/br/products/illustrator/).
Materials and methods
Study area
Manaus, the capital of Amazonas, has an area of 11,401,092 km2 and an estimated population of 2,182,763 inhabitants11. It is located in central Amazônia, the world’s largest tropical forest. The region has two seasons, a December-May rainy season with high volumes of rain (~30 cm per month) and a June-November dry season with little rain (~6 cm per month)24 (Fig. 2).
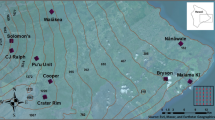
Locations of artificial larval Anopheles habitats in the Manaus periurban area. (a) Amazon River basin with the delineation of Amazonas state and Manaus; (b) sites, cover and land use; (c) composition of bands 8-4-3 for vegetation classification; (d) forest cover in 100 m buffers; (e) collection of limnological variables; (f) standard ladle for collecting larvae. The map was created using QGIS 3.16.5 (https://download.qgis.org).
Landscape analysis
We built non-overlapping buffers of 100 m in radius around 25 larval habitats that were created by human modifications (e.g., clay pits and ponds)20 and estimated the proportion of forest within each buffer (Fig. 2; Supplementary Table S1). Biological knowledge of species dispersal are used to support the spatial extent (buffer size) used in ecological and entomological studies25,26. Nonetheless, knowledge of dispersal movements of anopheline species is limited, so we used 100 m radii to avoid overlapping between neighbouring buffers. Moreover, 100 m represents an approximate mean of dispersion movement for some species27, which is important for relating dispersion of adult Anopheles from their larval habitats28. To classify land use, we used October 2016 images from the Sentinel-2 Level-1C sensor with a 10-m spatial resolution29. After processing the images, bands 3, 4 and 8 (green, red and near infrared respectively) were merged to perform a semi-automatic classification in Quantun GIS version 3.4.13 - Madeira, using the Plugin SCP (Semi automatic classification plugin) version 6.4.0 of Greenbelt. The classification resulted in binary data (e.g., forest or non-forest) and classification accuracy was tested via the SCP Plugin in Quantum GIS using Google Earth images as references.
Anopheles larval sampling
Larvae were collected in 2015 and 2016 one time each season for 30 min at each site by using a standard ladle with 350 mL volumetric capacity and a 1-m cable (Fig. 2 f). The larvae were fixed in McGregor solution and sent to the Laboratório de Malária e Dengue of the Instituto Nacional de Pesquisas da Amazônia (INPA) for identification. Collections were authorized under SISBIO permit 21264/5 and larvae were identified by using taxonomic keys30,31,32,33.
Abiotic variables
Water samples were collected in sterile flasks and sent to the Laboratório de Química Ambiental (INPA), for filtering, drying and weighing total suspended solids34. At each site, we used portable Orion pH 290A and YSI dissolved oxygen meters to measure pH, dissolved oxygen and water temperature in situ (Supplementary Fig. S1) (Fig. 2e). Daily precipitation amounts (mm) were obtained from the Manaus automatic meteorological station (BDMEP A101) of the National Institute of Meteorology35.
Data analyses
To compare rainfall (mm) between seasons, we first run a Shapiro-Wilk normality to select an appropriate analysis. Based on the non-normality of the data, we used the Kruskal-Wallis test. We also used two ordination techniques to summarize both environmental variables and anopheline larval assemblage composition. To summarize, the variance of environmental variables in a reduced space, we used Principal component analysis (PCA). The scores of the first two PCA axes that captured most of variation were used to depict gradients in environmental variables (predictors). Principal coordinate analysis (PCoA) was used to summarize anopheline larval composition into a low dimensional space. Unlike PCA, which preserves Euclidean distance between objects, PCoA ordinates objects on the basis of any resemblance index, which is more aproproate to count data. We used Hellinger distance as a dissimilarity measure of assemblage composition, which produces good representation of objects in ordination techiniques36. The scores of the two PCoA axes were used to represent variation in anopheline larval composition (response variables).
To compare rainfall (mm) between seasons, we used the Kruskal-Wallis test. The choice of this non-parametric analysis was based on the Shapiro-Wilk normality test. We also used two ordination techniques to summarize both environmental variables and anopheline larval assemblage composition. Principal component analysis (PCA) was used to summarize, in a low-dimension space, the variance of environmental variables into orthogonal axes; the scores of the first two PCA axes were used to depict gradients in environmental variables (predictors). We used principal coordinate analysis (PCoA) to summarize anopheline larval assemblage composition into orthogonal axes. Unlike PCA, which preserves Euclidean distance between objects, PCoA ordinates objects on the basis of any dissimilarity, allowing more flexible handling of ecological data, such as counts. We used Hellinger distance as a dissimilarity measure of assemblage composition, which is ideal for linear models. The scores of two PCoA axes were used as response variables.
Percent forest cover, PCA and PCoA axes were used to create a causal model (Fig. 1) that was tested using structural equation modeling (SEM)37. SEM is a useful framework for revealing causal relationships between predictor and response variables, explicitly including theory a priori (Fig. 1)38. In this framework, we compared patterns in the data to those implied by the a priori model, seeking to minimize difference between the model predictions and observed data. All relationships were modelled using Gaussian linear relationships. We used the maximum likelihood chi-square formula and the associated p-value to test model adequacy. The model would be considered suitable for our data when p > 0.05. Individual path coefficients were assessed using z-scores and p-values. The analyzes were performed in R, using the Lavaan package39,40.
Results
In the dry season, we collected 3474 individuals and 6603 individuals were collected in the rainy season. The most abundant species in both seasons were A. triannualtus (45.9 and 40.6%), A. darlingi (27.8 and 28.7%) and A. nuneztovari (9.4 and 13.5%) respectively. The malária vector, A. darlingi, was present in all larval habitats (Table 1).
Daily rainfall in the dry season ranged from 10.7 to 31.3 mm (mean = 26.6 ± 8.5 mm standard deviation) and in the rainy season, rainfall ranged from 235.3 to 303.9 mm (mean = 275.7 ± 29.7 standard deviation), indicating a significant seasonal difference in rainfall (Kruskal-Wallis, x2 = 37.857, df = 1, p<0.0001) (Supplementary Fig. S2).
The first two PCA axes explained 82% of the variation in water quality variables in the dry season and 80% in the rainy season. In both seasons, PCA1 captured a gradient of water quality, from sites with high dissolved oxygen concentration (negatively associated with PCA1) to those with high total suspended solids and pH (positively associated with PCA1). The most important PCA2 variable was water temperature (negatively associated in the dry season, positively associated in the rainy season) (Fig. 3).
The first two PCoA axes explained 52% of the variation in anopheline assemblages in the dry season and 60% in the rainy season. The most important species on the first axis in the dry season were A. nuneztovari (negatively associated) and A. nimbus (positively associated). In the rainy season, A. nuneztovari was also negatively associated with PCoA1, but A. peryassui, A. nimbus, A. braziliensis and A. triannulatus were positively associated with this axis. The most important species associated with PCoA2 in the dry season were A. oswaldoi, A. evansae, and A. nimbus (all negatively associated), but in the rainy season the most important species was A. nimbus (positively associated) (Fig. 4). Thus, there was a clear seasonal difference in anopheline larval assemblages.
PCoA of anopheline larval assemblage composition in 25 sites in Manaus during the dry (a) and rainy (b) season. A_nun: Anopheles nuneztovari; A_per: Anopheles peryassui; A_alb: Anopheles albitarsis; A_osw: Anopheles oswaldoi; A_eva: Anopheles evansae; A_tri: Anopheles triannulatus; A_bra: Anopheles braziliensis; A_dar: Anopheles darlingi; A_nim: Anopheles nimbus; Numbers are sites.
In both seasons, the SEMs supported our hypotheses (dry season: χ = 0.071; df = 1; p = 0.79; rainy season: χ = 0.000; df = 1; p = 0.98) (Fig. 5). Forest cover negatively affected PC1 in the dry season (coefficient= − 0.542, z= − 3.227, p= 0.001, R2 = 0.294), meaning that increased forest cover was associated with decreased values along PC1 (sites with more dissolved oxygen). PC1 was negatively associated with PCoA1 (coefficient= − 0.473, z= − 2.179, p= 0.029), but did not affect PCoA2 (coefficient= 0.014, z= 0.062, p= 0.951). The PCoA1-PCA1 relationship means that greater levels of total suspended solids and pH were associated with increased numbers of A. nuneztovati, A. peryassui, A. albitarsis, whereas higher dissolved oxygen levels were associated with increased abundance of A. nimbus. The direct effect of forest cover on PCoA1 was insignificant (coefficient= − 0.285, z= − 1.307, p= 0.191), and the indirect effect of forest cover via PC1 was marginally significant (coefficient= 0.264, z= 1.834, p= 0.067). Forest cover did not affect PC2 (coefficient= 0.082, z= 0.414, p= 0.679), and PC2 did not affected PCoA1 (coefficient= 0.092, z= 0.500, p= 0.617) or PCoA2 (coefficient= 0.053, z= − 0.269, p= 0.788). The direct effect and indirect effects of forest cover via PC2 on PCoA2 were also insignificant (direct: coefficient= − 0.145, z= − 0.617, p= 0.537; indirect: coefficient= 0.021, z= − 0.093, p= 0.926). Variables used in this SEM explained 17% of PCoA1 and 3% of PCoA2 (Fig. 5a).
Schematic representation of dry (a) and rainy (b) season SEM results. The continuous arrows indicate significant paths and the dashed arrows indicate insignificant paths; PC1 and PC2 are the scores of the ordination of site water quality variables and PCoA1 and PCoA2 represent larval anopheline assemblage composition captured by PCoA. R2 values are reported for each endogenous variable and non-standardized and standardized (in parentheses) coefficients are indicated on each path. The image was created using Abode Illustrator 2020 (https://www.adobe.com/br/products/illustrator/).
For the rainy season, forest cover had a significant negative effect on PC1, i.e., increased forest cover was associated with decreased PC1 values (sites with more dissolved oxygen) (coefficient= − 0.072, z= − 3.972; p= 0.000; R2 = 0.387). Both direct and indirect effects (via PC1) of forest cover positively affected PCoA1 (direct: coefficient= 0.451, z= 3.853; p= 0.000; indirect: coefficient= 0.332, z= 2.973, p= 0.003). PC1 was negatively associated with PCoA1 (coefficient= − 0.533, z= − 4.561, p= 0.000), but did not affect PCoA2 (coefficient= 0.199, z= 0.837, p= 0.403). This relationship between PCoA1 and PC1 indicated that larger concentrations of dissolved oxygen increased the number of A. nimbus, A. triannulatus, A. braziliensis, A. peryassui, whereas greater values of total suspended solids, pH and water temperature increased the number of A. nuneztovari and A. darlingi. Forest cover did not affect PC2 (coefficient= − 0.005, z= − 0.025, p= 0.980), and PC2 did not affect PCoA1 or PCoA2 respectively (coefficient= 0.063, z= 0.688, p= 0.492; coefficient= − 0.297, z= − 1.597, p= 0.110). The direct effect of forest cover on PCoA2 was also insignificant (direct: coefficient= 0.276, z= 1.163, p= 0.245), as was its indirect effect (indirect: coefficient= − 0.425, z= − 1.498, p= 0.134). Variables used in this SEM explained 79% of PCoA1 and 13% of PCoA2 (Fig. 5b). In summary, pH, dissolved oxygen and total suspended solids (but not temperature) affected anopheline assemblages in both seasons. Forest cover directly and indirectly affected anopheline assemblages in the rainy season, and had a marginal and indirect effect on anopheline assemblages in the dry season.
Discussion
We untangled how the direct and indirect paths of forest cover and water quality variables interact and shape anopheline assemblages in two seasons. Although previous studies determined how environmental variables at different spatial extents affected anopheline distributions in Amazônia, most studies focused on a single effect of an environmental variable or focused on single habitat types (terrestrial or aquatic)22,23,41,42. Our most important finding is that seasonality modulates the direct and indirect effects of forest cover on Amazônian anopheline larval distributions. In particular, we found that forest cover had stronger direct and indirect influence on larval anopheline assemblage composition in the rainy season than the dry season.
The different paths and strengths of forest cover influences on anopheline assemblages during the rainy and dry seasons can be associated with the responses of adults and larvae to forest characteristics. Forest cover influences water quality variables of ponds by shading, organic matter inputs and erosion processes43. These effects have consequences for pond water quality44 and favor the establishment of different culicid species45. We showed that during the rainy season, forest cover directly and indirectly influenced site water quality. Greater forest cover in the rainy season directly and indirectly affected A. nimbus and the secondary malaria vectors A. triannulatus and A. braziliensis positively. In the dry season, greater forest cover positively but marginally affected A. peryassui, A. nuneztovari and A. albitarsis, but only indirectly through water quality. Some species like A. triannulatus, A. nuneztovari and A. braziliensis coexist with the malaria vector, A. darlingi, in breeding sites46, and these species have been positively associated with pH, dissolved oxygen and total suspended solids in natural and artificial habitats20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47, which are environmental conditions favored by greater forest cover. The marginal indirect effect of forest cover on anopheline assemblage in the dry season suggests that we need caution in the interpretation of this result and long-term temporal data is required to confirm if this effect is corroborated.
Forest conditions influence mosquito vectors and their hosts. For example, some mosquitoes are zoophiles that feed on the blood of birds, reptiles, and mammals48, which are often more abundant in conserved areas. Other species are anthropophilic and prefer to feed on human blood49 and altered environments can force these species to migrate and, consequently, to change hosts48. In our study, A. triannulatus and A. minbus were more abundant in sites with more natural characteristics, whereas A. darlingi and A. nuneztovari were more abundant in altered landscapes. In addition, urbanization and deforestation increase the proximities of humans and domestic animals to mosquito vectors and their hosts, thereby maintaining and increasing transmission cycles50.
Forest conditions influence anopheline diversity by different paths, which may alter the strength of their seasonal effects. During the dry season, mosquito survival is also affected by altered microclimate (e.g., lower humidity)51 and lentic habitats contain less water, increased nutrient concentrations and decreased abundance and richness of mosquitoes52,53. We observed that rainfall plays an important role in the larval abundance of Anopheles in artificial larval habitats in Manaus. In addition, climatic factors such as rainfall and river levels are strongly associated with vector abundance and malaria cases in the region54,55. During the rainy season, increased water volume in artificial habitats provides more areas for distribution and development of mosquito species56 and we detected a significant increase in abundance of A. triannulatus, A. darlingi and A. nuneztovari. These observations may partially explain why we found a direct effect of forest cover on mosquitoes only during the rainy season.
Our results add more evidence that managing and conserving forest cover is important to control anophelines, thereby decreasing the contact of potential vectors (e.g., A. darlingi) with humans. In general, our results support the idea that mosquitoes are directly affected by the loss of native forest cover57 in the rainy season. Mosquitoes associated with serious human diseases (e.g., malaria, yellow fever, dengue, leishmaniasis) are more abundant in areas with low levels of native forest cover14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58. This is a critically important finding because recent studies have shown that forest cover plays an important role in the vector dynamics of mosquitoes and forest conservation keeps pathogens within the forest, avoiding spillover to human settlements59. On the other hand, deforestation provides favorable conditions for these vectors, thereby increasing malária cases and decreasing scores of the Human Development Index60. In addition, there is a positive correlation between mosquito abundance in fragmented forests and the prevalence of Plasmodium, the protozoan that causes malaria61.
Artificial larval habitats promote conditions for malaria vectors in Amazônia62,63. Therefore, the best way to develop control techniques would be to understand larval ecology in these habitats, where they are more sensitive to infections by pathogens, parasites, predation, larvicides and growth regulators64. This information is necessary to minimize failures in programs to control or eradicate the vector and the disease. Under this perspective, our study adds a new piece in the puzzle of mosquito control in Amazônia. For example, during the rainy season when forest cover directly and indirectly influences larval habitats, control programs can strengthen the control of key limnological variables, habitat structure, and entomological aspects, intensifying the environmental filter, particularly in areas with little forest cover and greater human concentrations near those habitats. The limnological study of Anopheles larval habitats is still far from complete, as each case has peculiarities inherent to them. Despite attempts, anophelines demonstrate versatility in relation to abiotic parameters20,21,22,23,24,25,26,65,66. However, we can use approaches that modify the larval environments. For example, more efficient management of water levels in fish farming ponds could decrease larval numbers and anopheline reproduction, Similarly, greater rationing of fish feed would decrease the supply of food resources for mosquito larvae. It is also worth mentioning that some variables are related to the efficiency of others. Regarding biological control via entomopathogenic bacteria, environmental factors (solar radiation) and water quality (amounts of total suspended solids and organic matter), can interfere with the effectiveness of the formulated Bacillus sphaericus applied in habitats for vector control62,63,64,65,66,67. Furthermore, eutrophication decreased the assemblages of aquatic invertebrates predating mosquito larvae.
Another alternative is the use of physical control (removal of grasses and macrophytes from the edge of habitats), helping to reduce microhabitats that provide larval refuges. Also, increased light and water temperature at the edges favor natural predation and biological control processes from potential fish and macroinvertebrates. The conservation of natural enemies and the use of biotic agents in the population control of vector mosquitoes have been recommended in small and medium-sized natural and artificial breeding sites19,20,21,22,23,24,25,26,27,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53. A combination of techniques that shape the important environmental variables for the establishment of these species are essential for vector control.
The analytical approach used here opens some windows of opportunity for improvements that are important to be recognized. First, our model did not incorporate important complexity of natural systems, such as ecological interactions among vectors and hosts, including human behavior. Agent-based models, including different host behavior, could provide important insights in this way. Second, our study is very limited in terms of temporal climatic variability. Additional information is needed to better understand the effects of long-term changes in land-use, water quality and climate and their interactions with mosquito assemblages in the region, particularly considering an ecological-evolutionary perspective. Third, it is important to highlight that the magnitude of effects of the estimated drivers were not the same in the rainy and dry seasons. Also, they may not remain constant in coming decades, especially considering potential regional process on mosquito assemblages, such as spillover effects, mass effects and host changes. Fourth, our study was carried out in an area of Amazonia that has experienced, a relatively old land use conversion from forest to urban areas (urban expansion rate of around 12% per year for the past 34 years)68. Beginning in the 1970s, human population increased at a rate of around 23% per decade and 25% in Manaus11. Therefore, the region we studied is very relevant in terms of historical interactions among human populations, mosquitoes and land use changes. However, understanding the effect of these changes on mosquito assemblages in areas with different land-use change dynamics, provides us with important information69, particularly those with very rapid urbanization processes, such as in the Arch of Deforestation70. Lastly, we need studies that consider the nexus among climate and land use changes, human and animal population health, economic conditions, and ecosystem services provided by these forest-urban transitional regions. Such information would facilitate including mosquito information in land use planning and climate mitigation programs based on forest management in and around cities.
Therefore, identifying ecological factors and paths that affect the composition of species of epidemiological importance are essential because they inform vector integrated management strategies. We emphasize that larval control in lentic habitats requires knowledge about larval ecology and the effects of biotic and abiotic variables on larvae, especially when it comes to biological controls. The application of integrated pest management can be conducted in both dry and rainy seasons. However, we recommend focusing on the dry season when larval habitats are more limited, in smaller volumes and more accessible for entry and application of vector control techniques. These are critically important considerations because over 2 million people live in Amazonas state11 and anophelines transmitted over 59,637 malaria cases in the Amazon region in the first half of 2020, and about 44.4% came from the state of Amazonas71.
References
Achard, F. et al. Determination of deforestation rates of the world’s humid tropical forests. Science 297, 999–1002. https://doi.org/10.1126/science.1070656 (2002).
Malhi, Y. et al. Climate change, deforestation, and the fate of the Amazon. Science 319, 169–172. https://doi.org/10.1126/science.1146961 (2008).
Laurance, W. F. et al. An Amazonian rainforest and its fragments as a laboratory of global change. Biol. Rev. 93, 223–247. https://doi.org/10.1111/brv.12343 (2018).
Barlow, J. et al. The future of hyperdiverse tropical ecosystems. Nature 559, 517–526. https://doi.org/10.1038/s41586-018-0301-1 (2018).
Steiger, D. B. M., Ritchie, S. A. & Laurance, S. G. W. Mosquito communities and disease risk influenced by land use change and seasonality in the Australian tropics. Parasites Vectors. 9, 387. https://doi.org/10.1186/s13071-016-1675-2 (2016).
Tadei, W. P. et al. Adaptative processes, control measures, genetic background, and resilience of malaria vectors and environmental changes in the Amazon region. Hydrobiology 789, 179–196. https://doi.org/10.1007/s10750-016-2960-y (2017).
World Health Organization. Vector-Borne Diseases. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (2020).
Fundação Oswaldo Cruz. Malária: Região Amazônica Concentra 99% dos casos no Brasil. https://portal.fiocruz.br/noticia/malaria-regiao-amazonica-concentra-99-dos-casos-no-brasil (2019).
Fundação de Vigilância em Saúde do Amazonas. Mais de 63 mil casos de malária foram registrados no Amazonas em 2019. http://www.fvs.am.gov.br/noticias_view/22 (2020).
Richards, P. & VanWey, L. Where deforestation leads to urbanization: How resource extraction is leading to urban growth in the Brazilian Amazon. Ann. Assoc. Am. Geogr. 105, 806–823. https://doi.org/10.1080/00045608.2015.1052337 (2015).
Instituto Brasileiro de Geografia e Estatística. Cidades e Estados. https://cidades.ibge.gov.br/brasil/am/manaus/panorama (2020).
Chase, J. Rainforest cities: Urbanization, development, and globalization of the Brazilian Amazon. J. Plan. Educ. Res. 18, 91–92. https://doi.org/10.1177/0739456x9801800114 (1998).
Sathler, D., Monte-Mór, R. L. & Carvalho, J. A. M. As redes para além dos rios: Urbanização e desequilíbrios na Amazônia brasileira. Nova Econ. 19, 11–39 (2009).
Santos, A. S. & Almeida, A. N. The impact of deforestation on malaria infections in the Brazilian Amazon. Ecol. Econ. 154, 247–256. https://doi.org/10.1016/j.ecolecon.2018.08.005 (2018).
MacDonald, A. J. & Mordecai, E. A. Amazon deforestation drives malaria transmission, and malaria burden reduces forest clearing. Proc. Natl. Acad. Sci. USA 116, 22212–22218. https://doi.org/10.1073/pnas.1905315116 (2019).
Pina-Costa, A. et al. Malaria in Brazil: What happens outside the Amazonian endemic region. Mem. Inst. Oswaldo Cruz. 109, 618–634. https://doi.org/10.1590/0074-0276140228 (2014).
Murdock, C. C., Evans, M. V., McClanahan, T. D., Miazgowicz, K. L. & Tesla, B. Fine-scale variation in microclimate across an urban landscape shapes variation in mosquito population dynamics and the potential of Aedes albopictus to transmit arboviral disease. PLoS Negl. Trop. Dis. 11, e0005640. https://doi.org/10.1371/journal.pntd.0005640 (2017).
Pongsiri, M. J. et al. Biodiversity loss affects global disease ecology. Bioscience 59, 945–954. https://doi.org/10.1525/bio.2009.59.11.6 (2009).
Ferreira, F. A. S., Arcos, A. N., Sampaio, R. T. M., Rodrigues, I. B. & Tadei, W. P. Effect of Bacillus sphaericus Neide on Anopheles (Diptera: Culicidae) and associated insect fauna in fish ponds in the Amazon. Rev. Bras. Entomol. 59, 234–239. https://doi.org/10.1016/j.rbe.2015.03.013 (2015).
Arcos, A. N., Ferreira, F. A. S., Cunha, H. B. & Tadei, W. P. Characterization of artificial larval habitats of Anopheles darlingi (Diptera: Culicidae) in the Brazilian Central Amazon. Rev. Bras. Entomol. 62, 267–274. https://doi.org/10.1016/j.rbe.2018.07.006 (2018).
Costa, M. R. A. et al. Extreme drought favors potential mixotrophic organisms in tropical semi-arid reservoirs. Hydrobiology 831, 43–54. https://doi.org/10.1007/s10750-018-3583-2 (2019).
Moreno, J. E., Rubio-Palis, Y., Sánchez, V. & Martínez, Á. Caracterización de hábitats larvales de anofelinos en el municipio Sifontes del estado Bolívar, Venezuela. Bol. Mal. Salud Amb. 55, 117–131 (2015).
Barros, V. L. L. et al. Study of behavioral patterns and infection analyses in anopheline species involved in the transmission of malaria in Buriticupu and São José de Ribamar municipality, Maranhão State, Brazil. EntomoBrasilis 13, e0820. https://doi.org/10.12741/ebrasilis.v13.e0820 (2020).
Farias, C. S., Veiga, J. A. P., Oliveira, E. & Queiroz, M. R. Análise do evento extremo chuvoso de 30 de setembro de 2013 ocorrido na cidade de Manaus. Ciênc. Nat. 39, 436–450. https://doi.org/10.5902/2179460X22693 (2017).
Miguet, P., Jackson, H. B., Jackson, N. D., Martin, A. E. & Fahrig, L. What determines the spatial extent of landscape effects on species?. Landsc. Ecol. 31, 1177–1194. https://doi.org/10.1007/s10980-015-0314-1 (2016).
Forattini, O. P. Culicidologia Médica 2nd edn, Vol. 860 (Edusp, 2002).
Greenberg, J. A., DiMenna, M. A., Hanelt, B. & Hofkin, B. V. Analysis of post-blood meal flight distances in mosquitoes utilizing zoo animal blood meals. J. Vector Ecol. 37, 83–89. https://doi.org/10.1111/j.1948-7134.2012.00203.x (2012).
Yakob, L. & Yan, G. A network population model of the dynamics and control of African malaria vectors. Trans. R. Soc. Trop. Med. Hyg. 104, 669–675. https://doi.org/10.1016/j.trstmh.2010.07.014 (2010).
ESA. Sentinel-2. https://sentinels.copernicus.eu/documents/247904/1848117/Sentinel-2_Data_Products_and_Access (2015).
Gorham, J. R., Stojanovich, C. J. & Scott, H. G. Clave ilustrada Para los Mosquitos Anofelinos de Sudamerica Oriental. (U.S. Department of Health, Education & Welfare, 1967).
Faran, M. E. Mosquito studies (Diptera: Culicidae). XXXIV. A revision of the Albimanus section of the subgenus Nyssorhynchus of Anopheles. Contr. Am. Entomol. Inst. 15, 1–215 (1980).
Faran, M. E. & Linthicum, K. J. A handbook of the amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culcidae). Mosq. Syst. 13, 1–81 (1981).
Consoli, R. A. G. B. & Lourenço-de-Oliveira, R. Principais Mosquitos de Importância Sanitária no Brasil (Fiocruz, 1994).
American Public Health Association. Standart Methods of the Experimination of Water and Wasterwater (American Public Health Association, 1985).
Instituto Nacional de Meteorologia. Banco de dados Meteorológicos Para Ensino e Pesquisa–BDMEP. http://www.inmet.gov.br/portal/index.php?r=bdmep/bdmep (2020).
Legendre, P. & Gallagher, E. D. Ecologically meaningful transformations for ordination of species data. Oecologia 129, 271–280. https://doi.org/10.1007/s004420100716 (2001).
Grace, J. B. Structural Equation Modeling and Natural Systems (Cambridge University Press, 2006).
Grace, J. B., Anderson, T. M., Olff, H. & Scheiner, S. M. On the specification of structural equation models for ecological systems. Ecol. Monogr. 80, 67–87. https://doi.org/10.1890/09-0464.1 (2010).
Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 48, 1–36. https://doi.org/10.18637/jss.v048.i02 (2012).
R CoreTeam. R: A Language and Environment for Statistical computing. (R Foundation for Statistical Computing, 2016). https://www.r-project.org/.
Villarreal-Treviño, C. et al. Composition and abundance of anopheline species according to habitat diversity in México. Salud Publ. Mex. 62, 388–401. https://doi.org/10.21149/10111 (2020).
Franklinos, L. H. V., Jones, K. E., Redding, D. W. & Abubakar, I. The effect of global change on mosquito-borne disease. Lancet Infect. Dis. 19, e302–e312. https://doi.org/10.1016/S1473-3099(19)30161-6 (2019).
Birkinshaw, S. J., Bathurst, J. C., Iroumé, A. & Palacios, H. The effect of forest cover on peak flow and sediment discharge—an integrated field and modelling study in central–southern Chile. Hydrol. Process. 25, 1284–1297. https://doi.org/10.1002/hyp.7900 (2011).
Arcos, A. N. et al. Water quality of urban lakes in the Central-Southern Region of Manaus, Amazon. Sci. Amazon. 7, CAm1–CAm11 (2018).
Emidi, B., Kisinza, W. N., Mmbando, B. P., Malima, R. & Mosha, F. W. Effect of physicochemical parameters on Anopheles and Culex mosquito larvae abundance in different breeding sites in a rural setting of Muheza, Tanzania. Parasites Vectors 10, 304. https://doi.org/10.1186/s13071-017-2238-x (2017).
Tadei, W. P. & Dutary-Thatcher, B. Malaria vectors in the Brazilian Amazon: Anopheles of the subgenus Nyssorhynchus. Rev. Inst. Med. Trop. S. Paulo. 42, 87–94. https://doi.org/10.1590/S0036-46652000000200005 (2000).
Tadei, W. P. et al. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am. J. Trop. Med. Hyg. 59, 325–335. https://doi.org/10.4269/ajtmh.1998.59.325 (1998).
Vinod, S. Deforestation and water pollution impact on mosquitoes related epidemic diseases in nanded region. Biosci. Discov. 2, 309–316 (2011).
Scott, T. W. & Takken, W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 28, 114–121. https://doi.org/10.1016/j.pt.2012.01.001 (2012).
Rückert, C. & Ebel, G. D. How do virus-mosquito interactions lead to viral emergence?. Trends Parasitol. 34, 310–321. https://doi.org/10.1016/j.pt.2017.12.004 (2018).
Afrane, Y. A., Zhou, G., Lawson, B. W., Githeko, A. K. & Yan, G. Effects of microclimatic changes caused by deforestation on the survivorship and reproductive fitness of Anopheles gambiae in western Kenya highlands. Am. J. Trop. Med. Hyg. 74, 772–778. https://doi.org/10.4269/ajtmh.2006.74.772 (2006).
Mattah, P. A. D. et al. Diversity in breeding sites and distribution of Anopheles mosquitoes in selected urban areas of southern Ghana. Parasites Vectors. 10, 25. https://doi.org/10.1186/s13071-016-1941-3 (2017).
Ferreira, F. A. Z. et al. Effects of diflubenzuron on associated insect fauna with Anopheles (Diptera: Culicidae) in laboratory, partial-field, and field conditions in the Central Amazon. An. Acad. Bras. Ciênc. 92, e20180590. https://doi.org/10.1590/0001-3765202020180590 (2020).
Galardo, A. K. R. et al. Seasonal abundance of anopheline mosquitoes and their association with rainfall and malaria along the Matapi River, Amapí, Brazil. Med. Vet. Entomol. 23, 335–349. https://doi.org/10.1111/j.1365-2915.2009.00839.x (2009).
Wolfarth-Couto, B., Silva, R. A. D. & Filizola, N. Variabilidade dos casos de malária e sua relação com a precipitação e nível d’água dos rios no Estado do Amazonas, Brasil. Cad. Saúde Pública. 35, e00020218. https://doi.org/10.1590/0102-311X00020218 (2019).
Coutinho, P. E. G., Candido, L. A., Tadei, W. P., Silva Junior, U. L. & Correa, H. K. M. An analysis of the influence of the local effects of climatic and hydrological factors affecting new malaria cases in riverine areas along the Rio Negro and surrounding Puraquequara Lake, Amazonas, Brazil. Environ. Monit. Assess. 190, 311. https://doi.org/10.1007/s10661-018-6677-4 (2018).
Ferraguti, M. et al. Effects of landscape anthropization on mosquito community composition and abundance. Sci. Rep. 6, 29002. https://doi.org/10.1038/srep29002 (2016).
World Health Organization. Vector-Borne Diseases. A Global Brief on Vector-Borne Diseases (No. WHO/DCO/WHD/2014.1). https://apps.who.int/iris/bitstream/handle/10665/111008/WHO_DCO_WHD_2014.1_eng.pdf (2014).
Ellwanger, J. H. et al. Beyond diversity loss and climate change: Impacts of Amazon deforestation on infectious diseases and public health. An. Acad. Bras. Ciênc. 92, e20191375. https://doi.org/10.1590/0001-3765202020191375 (2020).
Chaves, L. S. M., Conn, J. E., López, R. V. M. & Sallum, M. A. M. Abundance of impacted forest patches less than 5 km 2 is a key driver of the incidence of malaria in Amazonian Brazil. Sci. Rep. 8, 7077. https://doi.org/10.1038/s41598-018-25344-5 (2018).
Tchoumbou, M. A. et al. Effect of deforestation on prevalence of avian haemosporidian parasites and mosquito abundance in a tropical rainforest of Cameroon. Int. J. Parasitol. 50, 63–73. https://doi.org/10.1016/j.ijpara.2019.10.006 (2020).
Rodrigues, I. B., Tadei, W. P., Santos, R. L. C., Santos, S. & Baggio, J. B. Controle da Malária: Eficácia de formulados de Bacillus sphaericus 2362 contra larvas de espécies de Anopheles em criadouros artificiais: Tanques de piscicultura e Criadouros de Olaria. Rev. Patol. Trop. 37, 161–176 (2008).
Reis, I. C. et al. Diversity of Anopheles spp (Diptera: Culicidae) in an Amazonian urban area. Neotrop Entomol. 47, 412–417. https://doi.org/10.1007/s13744-018-0595-6 (2018).
Kamareddine, L. The biological control of the malaria vector. Toxins 4, 748–767. https://doi.org/10.3390/toxins4090748 (2012).
Arcos, A. N. et al. Diversidade de fitoplâncton em habitats aquáticos e conteúdo estomacal de larvas de Anopheles spp. (Diptera, Culicidae) em Manaus, Amazonas. In Ecologia, Evolução e Diversidade (ed. Luz, P. M.) 82–95 (Atena, 2018).
Laird, M. The Natural History of Larval Mosquito Habitas (London Academic Press, 1988).
Barjac, H. & Sutberland, D. J. Bacterial Control of Mosquitos e Black Flies: Biochemistry, Genetics e Applications of Bacillus thuringiensis israelensis and Bacillus sphaericus 343 (Rutgers University Press, 1990).
Souza, C. M. et al. Reconstructing three decades of land use and land cover changes in brazilian biomes with landsat archive and earth engine. Remote Sens. 12, 2735. https://doi.org/10.3390/rs12172735 (2020).
Norris, D. E. Mosquito-borne diseases as a consequence of land use change. EcoHealth 1, 19–24. https://doi.org/10.1007/s10393-004-0008-7 (2004).
Sathler, D., Adamo, S. B. & Lima, E. E. C. Deforestation and local sustainable development in Brazilian Legal Amazonia: An exploratory analysis. Ecol. Soc. 23, 30. https://doi.org/10.5751/ES-10062-230230 (2018).
Ministério da Saúde. Boletim Epidemiológico. https://www.gov.br/saude/pt-br/media/pdf/2020/dezembro/03/boletim_especial_malaria_1dez20_final.pdf (2020).
Acknowledgements
We thank the technicians of the Malaria and Dengue Laboratory and the Química Ambiental Laboratory of the Instituto Nacional de Pesquisas da Amazônia—INPA, and Fundação Universidade Federal de Mato Grosso do Sul—UFMS/MEC—Brazil, for their support in collections and analyzes. We also thank Carlos Praia and Gervilane Lima for the identification of specimens. A.N.A. received fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—“Finance Code 001” and from Fundacão de Apoio ao Desenvolvimento do Ensino, Ciência eTecnologia do Estado de Mato Grosso do Sul (FUNDECT). This study was financed in part by the Brazilian National Council for Scientific and Technological Development (CNPq), Grant nº 312998/2015-5—MCTIC/INPA/PCI. F.V.N. was supported by CAPES Grant 88882.317337/2019- 01. R.M.H. received a Fulbright Brasil grant.
Author information
Authors and Affiliations
Contributions
A.N.A., F.P.B., F.V.N., F.O.R. conceptualization the study, A.N.A., H.B.C., W.P.T. funding acquisition, A.N.A., F.A.S.F. investigation, A.N.A., F.P.B., F.V.N. formal analysis, and A.N.A., F.A.S.F., F.P.B., F.V.N., F.O.R., H.B.C., W.P.T., R.M.H. writing–review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arcos, A.N., Valente-Neto, F., da Silva Ferreira, F.A. et al. Seasonality modulates the direct and indirect influences of forest cover on larval anopheline assemblages in western Amazônia. Sci Rep 11, 12721 (2021). https://doi.org/10.1038/s41598-021-92217-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92217-9
This article is cited by
-
Spatial analyses of Plasmodium knowlesi vectors with reference to control interventions in Malaysia
Parasites & Vectors (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.