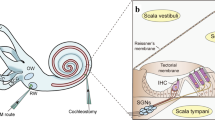
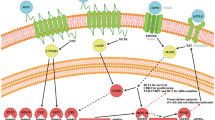
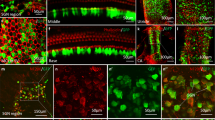
Abstract
Progress in deciphering the genetic architecture of human sensorineural hearing impairment (SNHI) or loss, and multidisciplinary studies of mouse models, have led to the elucidation of the molecular mechanisms underlying auditory system function, primarily in the cochlea, the mammalian hearing organ. These studies have provided unparalleled insights into the pathophysiological processes involved in SNHI, paving the way for the development of inner-ear gene therapy based on gene replacement, gene augmentation or gene editing. The application of these approaches in preclinical studies over the past decade has highlighted key translational opportunities and challenges for achieving effective, safe and sustained inner-ear gene therapy to prevent or cure monogenic forms of SNHI and associated balance disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Olusanya, B. O., Davis, A. C. & Hoffman, H. J. Hearing loss grades and the International Classification of Functioning, Disability and Health. Bull. World Health Organ. 97, 725–728 (2019).
Bussé, A. M. L. et al. Prevalence of permanent neonatal hearing impairment: systematic review and Bayesian meta-analysis. Int. J. Audiol. 59, 475–485 (2020).
WHO. World report on hearing. https://www.who.int/publications-detail-redirect/world-report-on-hearing (2021).
Lin, F. R. et al. Hearing loss and incident dementia. Arch. Neurol. 68, 214–220 (2011).
Panza, F., Solfrizzi, V. & Logroscino, G. Age-related hearing impairment — a risk factor and frailty marker for dementia and AD. Nat. Rev. Neurol. 11, 166–175 (2015).
Livingston, G. et al. Dementia prevention, intervention, and care. Lancet 390, 2673–2734 (2017).
Deal, J. A. et al. Hearing treatment for reducing cognitive decline: design and methods of the aging and cognitive health evaluation in elders randomized controlled trial. Alzheimers Dement. 4, 499–507 (2018).
Basner, M. et al. Auditory and non-auditory effects of noise on health. Lancet 383, 1325–1332 (2014).
Petit, C. Genes responsible for human hereditary deafness: symphony of a thousand. Nat. Genet. 14, 385–391 (1996). This review highlights the potential value of deciphering genetic architecture for elucidating the molecular mechanisms underlying cochlear development and physiology.
Richardson, G. P., de Monvel, J. B. & Petit, C. How the genetics of deafness illuminates auditory physiology. Annu. Rev. Physiol. 73, 311–334 (2011).
Giraudet, F. et al. Rapid exhaustion of auditory neural conduction in a prototypical mitochondrial disease, Friedreich ataxia. Clin. Neurophysiol. 129, 1121–1129 (2018).
Guilford, P. et al. A non-syndrome form of neurosensory, recessive deafness maps to the pericentromeric region of chromosome 13q. Nat. Genet. 6, 24–28 (1994).
Dib, C. et al. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380, 152–154 (1996).
Kelsell, D. P. et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387, 80–83 (1997).
Chan, D. K. & Chang, K. W. GJB2-associated hearing loss: systematic review of worldwide prevalence, genotype, and auditory phenotype. Laryngoscope 124, E34–E53 (2014).
Friedman, T. B. et al. A gene for congenital, recessive deafness DFNB3 maps to the pericentromeric region of chromosome 17. Nat. Genet. 9, 86–91 (1995).
Veske, A. et al. Autosomal recessive non-syndromic deafness locus (DFNB8) maps on chromosome 21q22 in a large consanguineous kindred from Pakistan. Hum. Mol. Genet. 5, 165–168 (1996).
Riazuddin, S. et al. Dominant modifier DFNM1 suppresses recessive deafness DFNB26. Nat. Genet. 26, 431–434 (2000).
Ansar, M. et al. A novel autosomal recessive non-syndromic deafness locus (DFNB35) maps to 14q24.1-14q24.3 in large consanguineous kindred from Pakistan. Eur. J. Hum. Genet. 11, 77–80 (2003).
Chaïb, H. et al. A gene responsible for a sensorineural nonsyndromic recessive deafness maps to chromosome 2p22-23. Hum. Mol. Genet. 5, 155–158 (1996).
Walsh, T. et al. From flies’ eyes to our ears: Mutations in a human class III myosin cause progressive nonsyndromic hearing loss DFNB30. Proc. Natl Acad. Sci. USA 99, 7518–7523 (2002).
Yasunaga, S. et al. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat. Genet. 21, 363–369 (1999).
Verpy, E. et al. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat. Genet. 26, 51–55 (2000).
Verpy, E. et al. Mutations in a new gene encoding a protein of the hair bundle cause non-syndromic deafness at the DFNB16 locus. Nat. Genet. 29, 345–349 (2001).
Zwaenepoel, I. et al. Otoancorin, an inner ear protein restricted to the interface between the apical surface of sensory epithelia and their overlying acellular gels, is defective in autosomal recessive deafness DFNB22. Proc. Natl Acad. Sci. USA 99, 6240–6245 (2002).
Weil, D. et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature 374, 60–61 (1995).
Gibson, F. et al. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature 374, 62–64 (1995).
Kurima, K. et al. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat. Genet. 30, 277–284 (2002).
Vreugde, S. et al. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat. Genet. 30, 257–258 (2002).
Bork, J. M. et al. Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am. J. Hum. Genet. 68, 26–37 (2001).
Li, X. C. et al. A mutation in PDS causes non-syndromic recessive deafness. Nat. Genet. 18, 215–217 (1998).
Avraham, K. B. et al. The mouse Snell’s waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat. Genet. 11, 369–375 (1995).
Wang, A. et al. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3. Science 280, 1447–1451 (1998).
Abu Rayyan, A. et al. Genomic analysis of inherited hearing loss in the Palestinian population. Proc. Natl Acad. Sci. USA 117, 20070–20076 (2020).
Denoyelle, F. et al. Clinical features of the prevalent form of childhood deafness, DFNB1, due to a connexin-26 gene defect: implications for genetic counselling. Lancet 353, 1298–1303 (1999).
Adadey, S. M. et al. Connexin genes variants associated with non-syndromic hearing impairment: a systematic review of the global burden. Life 10, E258 (2020).
D’Adamo, P. et al. Does epidermal thickening explain GJB2 high carrier frequency and heterozygote advantage? Eur. J. Hum. Genet. 17, 284–286 (2009).
Simpson, C., Kelsell, D. P. & Marchès, O. Connexin 26 facilitates gastrointestinal bacterial infection in vitro. Cell Tissue Res. 351, 107–116 (2013).
Leon, P. E., Raventos, H., Lynch, E., Morrow, J. & King, M. C. The gene for an inherited form of deafness maps to chromosome 5q31. Proc. Natl Acad. Sci. USA 89, 5181–5184 (1992).
Sheffield, A. M. & Smith, R. J. H. The epidemiology of deafness. Cold Spring Harb. Perspect. Med. 9, a033258 (2019).
Bolz, H. J. Hereditary hearing loss and its syndromes third edition. Eur. J. Hum. Genet. 24, 1650 (2016).
Weil, D. et al. The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nat. Genet. 16, 191–193 (1997).
Bitner-Glindzicz, M. et al. A recessive contiguous gene deletion causing infantile hyperinsulinism, enteropathy and deafness identifies the Usher type 1C gene. Nat. Genet. 26, 56–60 (2000).
Ahmed, Z. M. et al. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am. J. Hum. Genet. 69, 25–34 (2001).
Weil, D. et al. Usher syndrome type I G (USH1G) is caused by mutations in the gene encoding SANS, a protein that associates with the USH1C protein, harmonin. Hum. Mol. Genet. 12, 463–471 (2003).
Klimara, M. J. et al. De novo variants are a common cause of genetic hearing loss. Genet. Med. 24, 2555–2567 (2022).
Yan, D. et al. Mutation of the ATP-gated P2X(2) receptor leads to progressive hearing loss and increased susceptibility to noise. Proc. Natl Acad. Sci. USA 110, 2228–2233 (2013).
Brown, K. D. et al. Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 20, 1059–1068 (2014).
Delmaghani, S. et al. Hypervulnerability to sound exposure through impaired adaptive proliferation of peroxisomes. Cell 163, 894–906 (2015).
Gilels, F., Paquette, S. T., Beaulac, H. J., Bullen, A. & White, P. M. Severe hearing loss and outer hair cell death in homozygous Foxo3 knockout mice after moderate noise exposure. Sci. Rep. 7, 1054 (2017).
Mao, H. & Chen, Y. Noise-induced hearing loss: updates on molecular targets and potential interventions. Neural Plast. 2021, 4784385 (2021).
Van Eyken, E., Van Camp, G. & Van Laer, L. The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiol. Neurootol. 12, 345–358 (2007).
Bowl, M. R. & Dawson, S. J. Age-related hearing loss. Cold Spring Harb. Perspect. Med. 9, a033217 (2019).
Wells, H. R. R. et al. GWAS identifies 44 independent associated genomic loci for self-reported adult hearing difficulty in UK Biobank. Am. J. Hum. Genet. 105, 788–802 (2019).
Ivarsdottir, E. V. et al. The genetic architecture of age-related hearing impairment revealed by genome-wide association analysis. Commun. Biol. 4, 706 (2021).
Boucher, S. et al. Ultrarare heterozygous pathogenic variants of genes causing dominant forms of early-onset deafness underlie severe presbycusis. Proc. Natl Acad. Sci. USA 117, 31278–31289 (2020).
Tam, V. et al. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 20, 467–484 (2019).
Miao, L. et al. An overview of research trends and genetic polymorphisms for noise-induced hearing loss from 2009 to 2018. Environ. Sci. Pollut. Res. Int. 26, 34754–34774 (2019).
Lavinsky, J. et al. Genome-wide association study identifies nox3 as a critical gene for susceptibility to noise-induced hearing loss. PLoS Genet. 11, e1005094 (2015).
Chen, X.-M. et al. The role of genetic variants in the susceptibility of noise-induced hearing loss. Front. Cell Neurosci. 16, 946206 (2022).
Gallego-Martinez, A., Requena, T., Roman-Naranjo, P. & Lopez-Escamez, J. A. Excess of rare missense variants in hearing loss genes in sporadic Meniere disease. Front. Genet. 10, 76 (2019).
Richardson, G. P. & Petit, C. Hair-bundle links: genetics as the gateway to function. Cold Spring Harb. Perspect. Med. 9, a033142 (2019).
Safieddine, S., El-Amraoui, A. & Petit, C. The auditory hair cell ribbon synapse: from assembly to function. Annu. Rev. Neurosci. 35, 509–528 (2012).
Rutherford, M. A., von Gersdorff, H. & Goutman, J. D. Encoding sound in the cochlea: from receptor potential to afferent discharge. J. Physiol. 599, 2527–2557 (2021).
Zheng, J. et al. Prestin is the motor protein of cochlear outer hair cells. Nature 405, 149–155 (2000).
Lewis, M. A. et al. An ENU-induced mutation of miR-96 associated with progressive hearing loss in mice. Nat. Genet. 41, 614–618 (2009).
Mencía, A. et al. Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat. Genet. 41, 609–613 (2009).
Geng, R. et al. The microRNA-183/96/182 cluster is essential for stereociliary bundle formation and function of cochlear sensory hair cells. Sci. Rep. 8, 18022 (2018).
Avraham, K. B. et al. The noncoding genome and hearing loss. Hum. Genet. 141, 323–333 (2022).
Starr, A. & Rance, G. Auditory neuropathy. Handb. Clin. Neurol. 129, 495–508 (2015).
Kanzaki, S., Toyoda, M., Umezawa, A. & Ogawa, K. Application of mesenchymal stem cell therapy and inner ear regeneration for hearing loss: a review. Int. J. Mol. Sci. 21, 5764 (2020).
Bramhall, N. F., Shi, F., Arnold, K., Hochedlinger, K. & Edge, A. S. B. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Rep. 2, 311–322 (2014).
Lee, M. P. & Waldhaus, J. In vitro and in vivo models: what have we learnt about inner ear regeneration and treatment for hearing loss? Mol. Cell Neurosci. 120, 103736 (2022).
Ren, Y., Landegger, L. D. & Stankovic, K. M. Gene therapy for human sensorineural hearing loss. Front. Cell Neurosci. 13, 323 (2019).
Omichi, R., Shibata, S. B., Morton, C. C. & Smith, R. J. H. Gene therapy for hearing loss. Hum. Mol. Genet. 28, R65–R79 (2019).
Delmaghani, S. & El-Amraoui, A. Inner ear gene therapies take off: current promises and future challenges. J. Clin. Med. 9, E2309 (2020).
Farooq, R., Hussain, K., Tariq, M., Farooq, A. & Mustafa, M. CRISPR/Cas9: targeted genome editing for the treatment of hereditary hearing loss. J. Appl. Genet. 61, 51–65 (2020).
Bankoti, K. et al. Advances and challenges in adeno-associated viral inner-ear gene therapy for sensorineural hearing loss. Mol. Ther. Methods Clin. Dev. 21, 209–236 (2021).
Ding, N., Lee, S., Lieber-Kotz, M., Yang, J. & Gao, X. Advances in genome editing for genetic hearing loss. Adv. Drug Deliv. Rev. 168, 118–133 (2021).
Qi, J. et al. Current AAV-mediated gene therapy in sensorineural hearing loss. Fundam. Res. https://doi.org/10.1016/j.fmre.2022.08.015 (2022).
Chang, M. & Kanold, P. O. Development of auditory cortex circuits. J. Assoc. Res. Otolaryngol. 22, 237–259 (2021).
Meenderink, S. W. F., Shera, C. A., Valero, M. D., Liberman, M. C. & Abdala, C. Morphological immaturity of the neonatal organ of corti and associated structures in humans. J. Assoc. Res. Otolaryngol. 20, 461–474 (2019).
Lim, D. J. & Anniko, M. Developmental morphology of the mouse inner ear. A scanning electron microscopic observation. Acta Otolaryngol. Suppl. 422, 1–69 (1985).
Liberman, L. D. & Liberman, M. C. Postnatal maturation of auditory-nerve heterogeneity, as seen in spatial gradients of synapse morphology in the inner hair cell area. Hear. Res. 339, 12–22 (2016).
Babola, T. A. et al. Homeostatic control of spontaneous activity in the developing auditory system. Neuron 99, 511–524.e5 (2018).
Michalski, N. & Petit, C. Central auditory deficits associated with genetic forms of peripheral deafness. Hum. Genet. 141, 335–345 (2022).
Zhang, L. I., Bao, S. & Merzenich, M. M. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat. Neurosci. 4, 1123–1130 (2001).
Schreiner, C. E. & Polley, D. B. Auditory map plasticity: diversity in causes and consequences. Curr. Opin. Neurobiol. 24, 143–156 (2014).
Kirk, K. I. et al. Effects of age at implantation in young children. Ann. Otol. Rhinol. Laryngol. Suppl. 189, 69–73 (2002).
Kral, A., Dorman, M. F. & Wilson, B. S. Neuronal development of hearing and language: cochlear implants and critical periods. Annu. Rev. Neurosci. 42, 47–65 (2019).
Eggermont, J. J. Acquired hearing loss and brain plasticity. Hear. Res. 343, 176–190 (2017).
Pienkowski, M. & Eggermont, J. J. Cortical tonotopic map plasticity and behavior. Neurosci. Biobehav. Rev. 35, 2117–2128 (2011).
Lalwani, A. K., Walsh, B. J., Reilly, P. G., Muzyczka, N. & Mhatre, A. N. Development of in vivo gene therapy for hearing disorders: introduction of adeno-associated virus into the cochlea of the guinea pig. Gene Ther. 3, 588–592 (1996).
Salt, A. N. & Hirose, K. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear. Res. 362, 25–37 (2018).
Warchol, M. E. Interactions between macrophages and the sensory cells of the inner ear. Cold Spring Harb. Perspect. Med. 9, a033555 (2019).
Sherkow, J. S., Zettler, P. J. & Greely, H. T. Is it ‘gene therapy’? J. Law Biosci. 5, 786–793 (2018).
Takeda, H. et al. Prenatal electroporation-mediated gene transfer restores Slc26a4 knock-out mouse hearing and vestibular function. Sci. Rep. 9, 17979 (2019).
Geng, R. et al. Modeling and preventing progressive hearing loss in Usher syndrome III. Sci. Rep. 7, 13480 (2017).
Verdera, H. C., Kuranda, K. & Mingozzi, F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol. Ther. 28, 723–746 (2020).
Ivanchenko, M. V. et al. AAV-S: A versatile capsid variant for transduction of mouse and primate inner ear. Mol. Ther. Methods Clin. Dev. 21, 382–398 (2021).
György, B. et al. Rescue of hearing by gene delivery to inner-ear hair cells using exosome-associated AAV. Mol. Ther. 25, 379–391 (2017).
Akil, O. et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 75, 283–293 (2012). This work is the first to establish proof of concept for positive effects on hearing of gene replacement mediated by AAV1 in the inner ear of congenitally deaf mouse mutants.
Lee, J. et al. Efficient viral transduction in mouse inner ear hair cells with utricle injection and AAV9-PHP.B. Hear. Res. 394, 107882 (2020).
Yoshimura, H., Shibata, S. B., Ranum, P. T. & Smith, R. J. H. Enhanced viral-mediated cochlear gene delivery in adult mice by combining canal fenestration with round window membrane inoculation. Sci. Rep. 8, 2980 (2018).
Pan, B. et al. TMC1 Forms the pore of mechanosensory transduction channels in vertebrate inner ear hair cells. Neuron 99, 736–753.e6 (2018).
Jia, Y. et al. TMC1 and TMC2 proteins are pore-forming subunits of mechanosensitive ion channels. Neuron 105, 310–321.e3 (2020).
Akyuz, N. et al. Mechanical gating of the auditory transduction channel TMC1 involves the fourth and sixth transmembrane helices. Sci. Adv. 8, eabo1126 (2022).
Emptoz, A. et al. Local gene therapy durably restores vestibular function in a mouse model of Usher syndrome type 1G. Proc. Natl Acad. Sci. USA 114, 9695–9700 (2017). In this mouse model of a syndromic form of deafness, inner ear gene replacement results in a limited prevention of hearing impairment but persistent full rescue of the balance defect.
Pan, B. et al. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat. Biotechnol. 35, 264–272 (2017). In this mouse model of a syndromic form of deafness, inner-ear gene augmentation is shown to result in both substantial preventive effects on hearing impairment and the rescue of the balance defect persisting over several months.
Askew, C. et al. Tmc gene therapy restores auditory function in deaf mice. Sci. Transl. Med. 7, 295ra108 (2015).
Chien, W. W. et al. Gene therapy restores hair cell stereocilia morphology in inner ears of deaf whirler mice. Mol. Ther. 24, 17–25 (2016).
Akil, O. et al. Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model. Proc. Natl Acad. Sci. USA 116, 4496–4501 (2019). This study is the first to report reversion of congenital hearing impairment in a mouse model of human deafness by gene therapy inner-ear interventions performed after hearing onset.
Al-Moyed, H. et al. A dual-AAV approach restores fast exocytosis and partially rescues auditory function in deaf otoferlin knock-out mice. EMBO Mol. Med. 11, e9396 (2019).
Zhao, X., Liu, H., Liu, H., Cai, R. & Wu, H. Gene therapy restores auditory functions in an adult Vglut3 knockout mouse model. Hum. Gene Ther. 33, 729–739 (2022).
Isgrig, K. et al. Gene therapy restores balance and auditory functions in a mouse model of Usher syndrome. Mol. Ther. 25, 780–791 (2017).
Wu, J. et al. Single and dual vector gene therapy with AAV9-PHP.B rescues hearing in Tmc1 mutant mice. Mol. Ther. 29, 973–988 (2021).
Chang, Q. et al. Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange-Nielsen deafness syndrome. EMBO Mol. Med. 7, 1077–1086 (2015).
Nist-Lund, C. A. et al. Improved TMC1 gene therapy restores hearing and balance in mice with genetic inner ear disorders. Nat. Commun. 10, 236 (2019).
Michalski, N. et al. Harmonin-b, an actin-binding scaffold protein, is involved in the adaptation of mechanoelectrical transduction by sensory hair cells. Pflug. Arch. 459, 115–130 (2009).
Grillet, N. et al. Harmonin mutations cause mechanotransduction defects in cochlear hair cells. Neuron 62, 375–387 (2009).
Pascual-Garcia, P. & Capelson, M. Nuclear pores in genome architecture and enhancer function. Curr. Opin. Cell Biol. 58, 126–133 (2019).
Fierz, B. & Poirier, M. G. Biophysics of chromatin dynamics. Annu. Rev. Biophys. 48, 321–345 (2019).
Shivashankar, G. V. Mechanical regulation of genome architecture and cell-fate decisions. Curr. Opin. Cell Biol. 56, 115–121 (2019).
Nozawa, R.-S. & Gilbert, N. RNA: nuclear glue for folding the genome. Trends Cell Biol. 29, 201–211 (2019).
Ishino, Y., Krupovic, M. & Forterre, P. History of CRISPR-Cas from encounter with a mysterious repeated sequence to genome editing technology. J. Bacteriol. 200, e00580-17 (2018).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012). This study shows how the two RNAs of the CRISPR–Cas system direct Cas9-mediated target DNA cleavage at a specific site and establishes the possibility of engineering them as a single guide RNA.
Rouet, P., Smih, F. & Jasin, M. Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc. Natl Acad. Sci. USA 91, 6064–6068 (1994).
Jasin, M. & Rothstein, R. Repair of strand breaks by homologous recombination. Cold Spring Harb. Perspect. Biol. 5, a012740 (2013).
Chen, P. & Segil, N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 126, 1581–1590 (1999).
Jan, T. A. et al. Tympanic border cells are Wnt-responsive and can act as progenitors for postnatal mouse cochlear cells. Development 140, 1196–1206 (2013).
Lim, R. & Brichta, A. M. Anatomical and physiological development of the human inner ear. Hear. Res. 338, 9–21 (2016).
Zuris, J. A. et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 33, 73–80 (2015).
Gao, X. et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature 553, 217–221 (2018). This study is the first to demonstrate the editing of a deafness gene in a mouse model of human deafness using CRISPR–Cas9 editing to inactivate a mutant allele, significantly preventing the development of hearing loss.
György, B. et al. Allele-specific gene editing prevents deafness in a model of dominant progressive hearing loss. Nat. Med. 25, 1123–1130 (2019). In this study, CRISPR–Cas9 editing efficiency for a deafness gene in a mouse model of a human form of deafness is high, effectively preventing hearing loss at low frequencies over almost 1 year.
Zhao, Y. et al. A novel DFNA36 mutation in TMC1 orthologous to the Beethoven (Bth) mouse associated with autosomal dominant hearing loss in a Chinese family. PLoS ONE 9, e97064 (2014).
Noh, B. et al. In vivo outer hair cell gene editing ameliorates progressive hearing loss in dominant-negative Kcnq4 murine model. Theranostics 12, 2465–2482 (2022).
Xue, Y. et al. Gene editing in a Myo6 semi-dominant mouse model rescues auditory function. Mol. Ther. https://doi.org/10.1016/j.ymthe.2021.06.015 (2021).
Kohama, Y. et al. Adeno-associated virus-mediated gene delivery promotes S-phase entry-independent precise targeted integration in cardiomyocytes. Sci. Rep. 10, 15348 (2020).
Biehs, R. et al. DNA double-strand break resection occurs during non-homologous end joining in G1 but is distinct from resection during homologous recombination. Mol. Cell 65, 671–684.e5 (2017).
Shen, M. W. et al. Predictable and precise template-free CRISPR editing of pathogenic variants. Nature 563, 646–651 (2018).
Nishiguchi, K. M., Fujita, K., Miya, F., Katayama, S. & Nakazawa, T. Single AAV-mediated mutation replacement genome editing in limited number of photoreceptors restores vision in mice. Nat. Commun. 11, 482 (2020).
Liu, L. et al. Template-independent genome editing in the Pcdh15av-3j mouse, a model of human DFNB23 nonsyndromic deafness. Cell Rep. 40, 111061 (2022).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Nishida, K. et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 353, aaf8729 (2016).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019). This paper describes the highly versatile prime-editing technique, in which an engineered reverse transcriptase fused to a catalytically impaired Cas9 uses a guide RNA as a template for modifying single nucleotides or introducing indels in the target DNA, without generating double-strand DNA breaks.
Gaudelli, N. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Niggemann, P., György, B. & Chen, Z.-Y. Genome and base editing for genetic hearing loss. Hear. Res. 394, 107958 (2020).
Rees, H. A. et al. Improving the DNA specificity and applicability of base editing through protein engineering and protein delivery. Nat. Commun. 8, 15790 (2017).
Yeh, W.-H., Chiang, H., Rees, H. A., Edge, A. S. B. & Liu, D. R. In vivo base editing of post-mitotic sensory cells. Nat. Commun. 9, 2184 (2018).
Yeh, W.-H. et al. In vivo base editing restores sensory transduction and transiently improves auditory function in a mouse model of recessive deafness. Sci. Transl. Med. 12, eaay9101 (2020). This study reports the first proof of concept for the correction of a causal mutation of a deafness gene by base editing in an animal model of human deafness.
Chen, P. J. & Liu, D. R. Prime editing for precise and highly versatile genome manipulation. Nat. Rev. Genet. https://doi.org/10.1038/s41576-022-00541-1 (2022).
Ferreira da Silva, J. et al. Prime editing efficiency and fidelity are enhanced in the absence of mismatch repair. Nat. Commun. 13, 760 (2022).
Chen, P. J. et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 184, 5635–5652.e29 (2021).
Lentz, J. J. et al. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. Nat. Med. 19, 345–350 (2013).
Lentz, J. J. et al. Direct delivery of antisense oligonucleotides to the middle and inner ear improves hearing and balance in Usher mice. Mol. Ther. 28, 2662–2676 (2020).
Shibata, S. B. et al. RNA interference prevents autosomal-dominant hearing loss. Am. J. Hum. Genet. 98, 1101–1113 (2016).
Yoshimura, H., Shibata, S. B., Ranum, P. T., Moteki, H. & Smith, R. J. H. Targeted allele suppression prevents progressive hearing loss in the mature murine model of human TMC1 deafness. Mol. Ther. 27, 681–690 (2019).
Cox, D. B. T. et al. RNA editing with CRISPR-Cas13. Science 358, 1019–1027 (2017).
Zheng, Z. et al. Preventing autosomal-dominant hearing loss in Bth mice with CRISPR/CasRx-based RNA editing. Signal Transduct. Target. Ther. 7, 1–13 (2022).
Beurg, M. et al. Control of exocytosis by synaptotagmins and otoferlin in auditory hair cells. J. Neurosci. 30, 13281–13290 (2010).
Sun, S. et al. Hair cell mechanotransduction regulates spontaneous activity and spiral ganglion subtype specification in the auditory system. Cell 174, 1247–1263.e15 (2018).
Menchaca, A. et al. Otoferlin gene editing in sheep via CRISPR-assisted ssODN-mediated homology directed repair. Sci. Rep. 10, 5995 (2020).
Crispo, M. et al. Generation of a human deafness sheep model using the CRISPR/Cas system. Methods Mol. Biol. 2495, 233–244 (2022).
Carlson, R. J. et al. Association of genetic diagnoses of childhood-onset hearing loss with cochlear implant outcomes. JAMA Otolaryngol. Head. Neck Surg. https://doi.org/10.1001/jamaoto.2022.4463 (2023).
Burns, J. C., Cox, B. C., Thiede, B. R., Zuo, J. & Corwin, J. T. In vivo proliferative regeneration of balance hair cells in newborn mice. J. Neurosci. 32, 6570–6577 (2012).
Wang, T. et al. Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nat. Commun. 6, 6613 (2015).
Forge, A., Li, L., Corwin, J. T. & Nevill, G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science 259, 1616–1619 (1993).
Warchol, M. E., Lambert, P. R., Goldstein, B. J., Forge, A. & Corwin, J. T. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science 259, 1619–1622 (1993).
Paplou, V., Schubert, N. M. A. & Pyott, S. J. Age-related changes in the cochlea and vestibule: shared patterns and processes. Front. Neurosci. 15, 680856 (2021).
Oestreicher, D., Picher, M. M., Rankovic, V., Moser, T. & Pangrsic, T. Cabp2-gene therapy restores inner hair cell calcium currents and improves hearing in a DFNB93 mouse model. Front. Mol. Neurosci. 14, 689415 (2021).
Iizuka, T. et al. Perinatal Gjb2 gene transfer rescues hearing in a mouse model of hereditary deafness. Hum. Mol. Genet. 24, 3651–3661 (2015).
Wu, X. et al. Gene therapy via canalostomy approach preserves auditory and vestibular functions in a mouse model of Jervell and Lange-Nielsen syndrome type 2. Nat. Commun. 12, 697 (2021).
Kim, M.-A. et al. Methionine sulfoxide reductase B3-targeted in utero gene therapy rescues hearing function in a mouse model of congenital sensorineural hearing loss. Antioxid. Redox Signal. 24, 590–602 (2016).
Rankovic, V. et al. Overloaded adeno-associated virus as a novel gene therapeutic tool for otoferlin-related deafness. Front. Mol. Neurosci. 13, 600051 (2020).
Tang, H. et al. Hearing of Otof-deficient mice restored by trans-splicing of N- and C-terminal otoferlin. Hum. Genet. 142, 289–304 (2023).
Lu, Y.-C. et al. Gene therapy with a synthetic adeno-associated viral vector improves audiovestibular phenotypes in Pjvk-mutant mice. JCI Insight 7, e152941 (2022).
Kim, M.-A. et al. Gene therapy for hereditary hearing loss by SLC26A4 mutations in mice reveals distinct functional roles of pendrin in normal hearing. Theranostics 9, 7184–7199 (2019).
Tao, Y. et al. AAV-ie-K558R mediated cochlear gene therapy and hair cell regeneration. Signal. Transduct. Target. Ther. 7, 109 (2022).
Shubina-Oleinik, O. et al. Dual-vector gene therapy restores cochlear amplification and auditory sensitivity in a mouse model of DFNB16 hearing loss. Sci. Adv. 7, eabi7629 (2021).
Taiber, S. et al. Neonatal AAV gene therapy rescues hearing in a mouse model of SYNE4 deafness. EMBO Mol. Med. 13, e13259 (2021).
Dulon, D. et al. Clarin-1 gene transfer rescues auditory synaptopathy in model of Usher syndrome. J. Clin. Invest. 128, 3382–3401 (2018).
György, B. et al. Gene transfer with AAV9-PHP.B rescues hearing in a mouse model of Usher syndrome 3A and transduces hair cells in a non-human primate. Mol. Ther. Methods Clin. Dev. 13, 1–13 (2019).
Jiang, F. & Doudna, J. A. CRISPR-Cas9 structures and mechanisms. Annu. Rev. Biophys. 46, 505–529 (2017).
Shibata, S. B., Yoshimura, H., Ranum, P. T., Goodwin, A. T. & Smith, R. J. H. Intravenous rAAV2/9 injection for murine cochlear gene delivery. Sci. Rep. 7, 9609 (2017).
Yamasoba, T., Yagi, M., Roessler, B. J., Miller, J. M. & Raphael, Y. Inner ear transgene expression after adenoviral vector inoculation in the endolymphatic sac. Hum. Gene Ther. 10, 769–774 (1999).
de Lourdes Flores García, M., de la Llata Segura, C., Cisneros Lesser, J. C. & Pane Pianese, C. Endolymphatic sac surgery for Ménière’s disease — current opinion and literature review. Int. Arch. Otorhinolaryngol. 21, 179–183 (2017).
Li, C. & Samulski, R. J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 21, 255–272 (2020).
Tabebordbar, M. et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 184, 4919–4938.e22 (2021).
Ahmad, S., Chen, S., Sun, J. & Lin, X. Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Biochem. Biophys. Res. Commun. 307, 362–368 (2003).
Everett, L. A., Morsli, H., Wu, D. K. & Green, E. D. Expression pattern of the mouse ortholog of the Pendred’s syndrome gene (Pds) suggests a key role for pendrin in the inner ear. Proc. Natl Acad. Sci. USA 96, 9727–9732 (1999).
Ivanchenko, M. V. et al. Preclinical testing of AAV9-PHP.B for transgene expression in the non-human primate cochlea. Hear. Res. 394, 107930 (2020).
Andres-Mateos, E. et al. Choice of vector and surgical approach enables efficient cochlear gene transfer in nonhuman primate. Nat. Commun. 13, 1359 (2022).
Lai, Y. et al. Efficient in vivo gene expression by trans-splicing adeno-associated viral vectors. Nat. Biotechnol. 23, 1435–1439 (2005).
Akil, O. Dual and triple AAV delivery of large therapeutic gene sequences into the inner ear. Hear. Res. 394, 107912 (2020).
Li, J., Sun, W., Wang, B., Xiao, X. & Liu, X.-Q. Protein trans-splicing as a means for viral vector-mediated in vivo gene therapy. Hum. Gene Ther. 19, 958–964 (2008).
Ahmed, K. S. et al. Liposome: composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 27, 742–761 (2019).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science 367, eaau6977 (2020).
Meng, W. et al. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 27, 585–598 (2020).
Hudry, E. et al. Exosome-associated AAV vector as a robust and convenient neuroscience tool. Gene Ther. 23, 380–392 (2016).
György, B., Fitzpatrick, Z., Crommentuijn, M. H. W., Mu, D. & Maguire, C. A. Naturally enveloped AAV vectors for shielding neutralizing antibodies and robust gene delivery in vivo. Biomaterials 35, 7598–7609 (2014).
Cheng, M. et al. Neutralizing antibody evasion and transduction with purified extracellular vesicle-enveloped AAV vectors. Hum. Gene Ther. https://doi.org/10.1089/hum.2021.122 (2021).
Sato, Y. T. et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 6, 21933 (2016).
Lin, Y. et al. Exosome-liposome hybrid nanoparticles deliver CRISPR/Cas9 system in MSCs. Adv. Sci. 5, 1700611 (2018).
Chuang, Y.-F. et al. Approach for in vivo delivery of CRISPR/Cas system: a recent update and future prospect. Cell Mol. Life Sci. 78, 2683–2708 (2021).
Jeng, J.-Y. et al. AAV-mediated rescue of Eps8 expression in vivo restores hair-cell function in a mouse model of recessive deafness. Mol. Ther. Methods Clin. Dev. 26, 355–370 (2022).
Maeda, Y., Fukushima, K., Nishizaki, K. & Smith, R. J. H. In vitro and in vivo suppression of GJB2 expression by RNA interference. Hum. Mol. Genet. 14, 1641–1650 (2005).
Yu, Q. et al. Virally expressed connexin26 restores gap junction function in the cochlea of conditional Gjb2 knockout mice. Gene Ther. 21, 71–80 (2014).
Guo, J. et al. GJB2 gene therapy and conditional deletion reveal developmental stage-dependent effects on inner ear structure and function. Mol. Ther. Methods Clin. Dev. 23, 319–333 (2021).
Wang, L. et al. Fetal antisense oligonucleotide therapy for congenital deafness and vestibular dysfunction. Nucleic Acids Res. 48, 5065–5080 (2020).
Acknowledgements
The authors thank P. Avan for fruitful discussions on physiological aspects and N. Michalski, S. Delmaghani and J. Boutet de Monvel for critical reading of the manuscript. The authors also thank C. Calvet (IdA) for help with the figures and E. Gomard for assistance with cochlea drawings (Communication Institutionnelle et Image, Institut Pasteur). This work was supported by Fondation pour l’Audition (FPA IDA05 to CP; FPA IDA08 to SS), Ile de France (DIM Thérapie génique to C.P. and S.S.), EARGENCURE (ANR-17-CE18–0027 to S.S.), PRESAGE (ANR-21-CE14–0075 to C.P.), RHU AUDINNOVE (ANR-18-RHUS-0007 to C.P. and S.S.).
Author information
Authors and Affiliations
Contributions
C.P. and C.B. researched the literature. C.P. and S.S. wrote the article. All authors reviewed and/or edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks Zheng-Yi Chen, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Van Camp, G., Smith, R. J. H. Hereditary Hearing Loss Homepage: https://hereditaryhearingloss.org
Supplementary information
Glossary
- Adeno-associated virus
-
(AAV). Single-stranded DNA virus from which recombinant AAVs are engineered as vectors for gene therapy for DNA delivery to target cells.
- Antisense oligonucleotides
-
(ASOs). Short, synthetic, single-stranded sequences of 15–30 nucleotides designed to target other nucleotide sequences, usually coding or non-coding RNAs. They can mediate target RNA degradation, act as steric blockers and modulate exon splicing.
- Canalostomy
-
A surgical approach to the inner ear involving fenestration of a vestibule semicircular canal, generally the posterior semicircular canal; used in inner-ear gene therapy interventions in mice.
- Cochlear implant
-
A surgically implanted neuroprosthesis consisting of an electrical device that processes sounds and transforms acoustic stimulation into electrical stimulation delivered directly to the primary auditory neurons via electrodes inserted into the cochlea.
- Conductive
-
Hearing impairment resulting from the defective transmission of sound waves to the cochlea due to external and/or middle-ear defects.
- Dominant-negative variant
-
The product of a mutated gene carrying a variant with a dominant-negative effect has not only lost its function, but it also interferes in a deleterious manner with the function of the normal, wild-type gene product present within the same cell of heterozygous organisms.
- Episome
-
A genetic determinant (such as the DNA of some bacteriophages) that can replicate autonomously in bacterial cytoplasm or as an integral part of the chromosomes.
- Gene editing
-
Processes that change genomic DNA by adding, removing or altering sequences. Gene editing technologies produce site-specific modifications.
- Gene replacement
-
A procedure for correcting the effects of defective genes by transferring a normal copy of the gene into targeted diseased cells.
- Genetic architecture
-
The underlying genetic basis of the phenotypic features of a disease or other trait. It includes all the causal variants, the magnitude of their effects, their frequency and their interactions with each other and with environmental factors.
- Genome-wide association studies
-
(GWAS). A genetic approach for identifying genetic risk factors for complex diseases or traits, by scanning for genome-wide associations between single-nucleotide polymorphisms (SNPs) and phenotypes.
- Haploinsufficient gene
-
A gene that must have two normally functioning alleles for a normal phenotype.
- Hearing aid
-
An auditory prosthesis; specifically, an electrical device amplifying sound at selected frequencies.
- Hearing impairment
-
Used interchangeably with ‘deafness’ in this Review (deafness not being restricted to profound hearing impairment). Hearing impairment in humans is classified as mild, moderate, moderately severe, severe and profound, for threshold elevations ranging from 26–40 dB hearing level (HL), 41–55 dB HL, 56–70 dB HL, 71–90 dB HL and >90 dB HL, respectively, relative to normal human hearing thresholds.
- Hearing threshold
-
The minimal intensity a sound of a given frequency must reach to be detected; measured in dB SPL (sound pressure level), with 0 dB SPL corresponding to 20 µPa. In clinical context, thresholds are obtained at four frequencies of 0.5, 1, 2 and 4 kHz and are expressed in dB on the Hearing Level (dB HL) scale. 0 dB HL represents for each frequency the average auditory threshold of individuals with normal hearing.
- Heterochrony
-
Evolutionary change in timing and rates of developmental processes.
- Homozygosity mapping
-
A method to identify genomic regions containing causal mutations for recessively inherited diseases through homozygous polymorphic markers co-inherited with the disease. Homozygous regions are generally large for recent consanguineous unions and shorter in cases of ancestral population homozygosity.
- Meniere disease
-
A chronic inner-ear disorder characterized by episodes of vertigo (dizzy spells), tinnitus (perception of sound without an external sound stimulus) and hearing loss.
- Ribbon pre-synapse
-
An electron-dense structure present in the sensory cells of various sensory systems, to which synaptic vesicles are tethered. In the mature auditory system, presynaptic ribbons are present only in inner hair cells. Each ribbon pre-synapse faces the post-synaptic ending of the single dendrite of the primary afferent neuron.
- Small interfering RNA
-
Molecular effectors of RNA interference (RNAi). These double-stranded small RNAs, 19–25 bp long, can be used to inhibit transcription, degrade RNA and repress mRNA translation.
- SNP
-
(Single-nucleotide-polymorphism). A single-base genomic variant. A variant is considered to be a SNP if its frequency exceeds 1% in a large population of unrelated individuals.
- Stria vascularis
-
A highly specialized three-layered epithelium of the lateral wall of the cochlear duct housing a dense capillary network. The stria vascularis is involved in K+ secretion into the endolymph and the generation of endocochlear potential.
- Tonotopic
-
Expression of gradients in the representation (maps) of sound properties, such as their frequency-to-place representation, or frequency maps of pure tone stimuli, from the cochlea to the primary auditory cortex and in all the nuclei of the auditory sensory pathway in between.
- Usher syndrome
-
(USH). An autosomal recessively inherited multisensory disorder, with three clinical subtypes (USH1–3). The most severe subtype, USH1, combines congenital severe-to-profound sensorineural deafness, balance defects of vestibular origin and retinitis pigmentosa leading to blindness.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Petit, C., Bonnet, C. & Safieddine, S. Deafness: from genetic architecture to gene therapy. Nat Rev Genet 24, 665–686 (2023). https://doi.org/10.1038/s41576-023-00597-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-023-00597-7
This article is cited by
-
Inner Ear Organoids: Strengths and Limitations
Journal of the Association for Research in Otolaryngology (2024)
-
Treatment of monogenic and digenic dominant genetic hearing loss by CRISPR-Cas9 ribonucleoprotein delivery in vivo
Nature Communications (2023)