Abstract
Cardiovascular diseases (CVDs), such as ischaemic heart disease, cardiomyopathy, atherosclerosis, hypertension, stroke and heart failure, are among the leading causes of morbidity and mortality worldwide. Although specific CVDs and the associated cardiometabolic abnormalities have distinct pathophysiological and clinical manifestations, they often share common traits, including disruption of proteostasis resulting in accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER). ER proteostasis is governed by the unfolded protein response (UPR), a signalling pathway that adjusts the protein-folding capacity of the cell to sustain the cell’s secretory function. When the adaptive UPR fails to preserve ER homeostasis, a maladaptive or terminal UPR is engaged, leading to the disruption of ER integrity and to apoptosis. ER stress functions as a double-edged sword, with long-term ER stress resulting in cellular defects causing disturbed cardiovascular function. In this Review, we discuss the distinct roles of the UPR and ER stress response as both causes and consequences of CVD. We also summarize the latest advances in our understanding of the importance of the UPR and ER stress in the pathogenesis of CVD and discuss potential therapeutic strategies aimed at restoring ER proteostasis in CVDs.
Key points
-
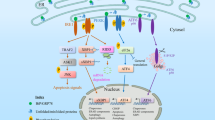
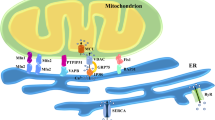
The endoplasmic reticulum (ER) regulates crucial processes governing cardiovascular function; ER and mitochondria contacts assist in the transport of mitochondrial Ca2+, and abnormalities cause cardiomyocyte mitochondrial dysfunction.
-
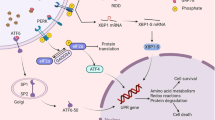
ER stress can be beneficial through an adaptive unfolded protein response (UPR) or detrimental through a maladaptive UPR; excessive ER stress perturbs the function of secretory pathways, contributing to cardiovascular pathology.
-
ER stress can be either a cause or a consequence of cardiovascular disease (CVD); disrupted ER homeostasis provokes the onset of CVD, which further exacerbates ER stress, creating a vicious cycle.
-
Canonical and non-canonical ER stress signalling can contribute to either cardiovascular protection or pathology, depending on the cellular environment and disease progression status.
-
Classic ER stress sensors (such as inositol-requiring protein 1α) also have a non-canonical function, such as scaffolding between cellular organelles.
-
Strategies to reduce ER stress (such as small-molecule proteostasis promoters and gene therapy) help to stabilize misfolded proteins and promote correct protein folding, thereby contributing to the prevention and management of CVD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hetz, C., Zhang, K. & Kaufman, R. J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 21, 421–438 (2020).
Pastor-Cantizano, N., Ko, D. K., Angelos, E., Pu, Y. & Brandizzi, F. Functional diversification of ER stress responses in Arabidopsis. Trends Biochem. Sci. 45, 123–126 (2019).
Lebeaupin, C. et al. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 69, 927–947 (2018).
Chen, Y. J., Quintanilla, C. G. & Liou, J. Recent insights into mammalian ER-PM junctions. Curr. Opin. Cell Biol. 57, 99–105 (2019).
Hwang, J. & Qi, L. Quality control in the endoplasmic reticulum: crosstalk between ERAD and UPR pathways. Trends Biochem. Sci. 43, 593–605 (2018).
Loi, M. & Molinari, M. Mechanistic insights in recov-ER-phagy: micro-ER-phagy to recover from stress. Autophagy 16, 385–386 (2020).
Zhou, H., Wang, S., Hu, S., Chen, Y. & Ren, J. ER-mitochondria microdomains in cardiac ischemia-reperfusion injury: a fresh perspective. Front. Physiol. 9, 755 (2018).
Guido, D., Demaurex, N. & Nunes, P. Junctate boosts phagocytosis by recruiting endoplasmic reticulum Ca2+ stores near phagosomes. J. Cell Sci. 128, 4074–4082 (2015).
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259 (2017).
Hong, J., Kim, K., Kim, J. H. & Park, Y. The role of endoplasmic reticulum stress in cardiovascular disease and exercise. Int. J. Vasc. Med. 2017, 2049217 (2017).
Diaz-Bulnes, P., Saiz, M. L., Lopez-Larrea, C. & Rodriguez, R. M. Crosstalk between hypoxia and ER stress response: a key regulator of macrophage polarization. Front. Immunol. 10, 2951 (2019).
Wang, S. et al. Endoplasmic reticulum stress in the heart: insights into mechanisms and drug targets. Br. J. Pharmacol. 175, 1293–1304 (2018).
Wang, X., Xu, L., Gillette, T. G., Jiang, X. & Wang, Z. V. The unfolded protein response in ischemic heart disease. J. Mol. Cell. Cardiol. 117, 19–25 (2018).
Glembotski, C. C. Endoplasmic reticulum stress in the heart. Circ. Res. 101, 975–984 (2007).
Groenendyk, J., Agellon, L. B. & Michalak, M. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu. Rev. Physiol. 75, 49–67 (2013).
Gonzalez-Teuber, V. et al. Small molecules to improve ER proteostasis in disease. Trends Pharmacol. Sci. 40, 684–695 (2019).
Vega, H., Agellon, L. B. & Michalak, M. The rise of proteostasis promoters. IUBMB Life 68, 943–954 (2016).
Valenzuela, V., Jackson, K. L., Sardi, S. P. & Hetz, C. Gene therapy strategies to restore ER proteostasis in disease. Mol. Ther. 26, 1404–1413 (2018).
Jin, J. K. et al. ATF6 decreases myocardial ischemia/reperfusion damage and links ER stress and oxidative stress signaling pathways in the heart. Circ. Res. 120, 862–875 (2017).
Chengji, W. & Xianjin, F. Exercise protects against diabetic cardiomyopathy by the inhibition of the endoplasmic reticulum stress pathway in rats. J. Cell. Physiol. 234, 1682–1688 (2019).
Wu, N. N. et al. Physical exercise and selective autophagy: benefit and risk on cardiovascular health. Cells 8, 1436 (2019).
Stolz, A., Ernst, A. & Dikic, I. Cargo recognition and trafficking in selective autophagy. Nat. Cell Biol. 16, 495–501 (2014).
Wang, M. & Kaufman, R. J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529, 326–335 (2016).
Jacquemyn, J., Cascalho, A. & Goodchild, R. E. The ins and outs of endoplasmic reticulum-controlled lipid biosynthesis. EMBO Rep. 18, 1905–1921 (2017).
Nishimura, T. & Stefan, C. J. Specialized ER membrane domains for lipid metabolism and transport. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 1865, 158492 (2020).
Di Scala, C. et al. Relevance of CARC and CRAC cholesterol-recognition motifs in the nicotinic acetylcholine receptor and other membrane-bound receptors. Curr. Top. Membr. 80, 3–23 (2017).
Brown, M. S., Radhakrishnan, A. & Goldstein, J. L. Retrospective on cholesterol homeostasis: the central role of scap. Annu. Rev. Biochem. 87, 783–807 (2018).
Eid, W. et al. mTORC1 activates SREBP-2 by suppressing cholesterol trafficking to lysosomes in mammalian cells. Proc. Natl Acad. Sci. USA 114, 7999–8004 (2017).
Widenmaier, S. B. et al. NRF1 Is an ER membrane sensor that is central to cholesterol homeostasis. Cell 171, 1094–1109 (2017).
Wilhelm, L. P. et al. STARD3 mediates endoplasmic reticulum-to-endosome cholesterol transport at membrane contact sites. EMBO J. 36, 1412–1433 (2017).
Goder, V., Alanis-Dominguez, E. & Bustamante-Sequeiros, M. Lipids and their (un)known effects on ER-associated protein degradation (ERAD). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1865, 158488 (2020).
Zhou, Z. et al. Endoplasmic reticulum-associated degradation regulates mitochondrial dynamics in brown adipocytes. Science 368, 54–60 (2020).
Saheki, Y. & De Camilli, P. Endoplasmic reticulum-plasma membrane contact sites. Annu. Rev. Biochem. 86, 659–684 (2017).
Zhihao, L. et al. SERCA2a: a key protein in the Ca(2+) cycle of the heart failure. Heart Fail. Rev. 25, 523–535 (2019).
Ureshino, R. P. et al. The interplay between Ca(2+) signaling pathways and neurodegeneration. Int. J. Mol. Sci. 20, 6004 (2019).
Collins, H. E. et al. Novel role of the ER/SR Ca(2+) sensor STIM1 in the regulation of cardiac metabolism. Am. J. Physiol. Heart Circ. Physiol. 316, H1014–H1026 (2019).
Eden, E. R. The formation and function of ER-endosome membrane contact sites. Biochim. Biophys. Acta 1861, 874–879 (2016).
Kilpatrick, B. S. et al. An endosomal NAADP-sensitive two-pore Ca(2+) channel regulates ER-endosome membrane contact sites to control growth factor signaling. Cell Rep. 18, 1636–1645 (2017).
Henne, W. M. et al. Mdm1/Snx13 is a novel ER-endolysosomal interorganelle tethering protein. J. Cell Biol. 210, 541–551 (2015).
Fameli, N., Ogunbayo, O. A., van Breemen, C. & Evans, A. M. Cytoplasmic nanojunctions between lysosomes and sarcoplasmic reticulum are required for specific calcium signaling. F1000Research 3, 93 (2014).
Ambudkar, I. S., de Souza, L. B. & Ong, H. L. TRPC1, Orai1, and STIM1 in SOCE: friends in tight spaces. Cell Calcium 63, 33–39 (2017).
Stefan, C. J., Manford, A. G. & Emr, S. D. ER-PM connections: sites of information transfer and inter-organelle communication. Curr. Opin. Cell Biol. 25, 434–442 (2013).
Chung, J. et al. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 349, 428–432 (2015).
Osterrieder, A. et al. Stacks off tracks: a role for the golgin AtCASP in plant endoplasmic reticulum-Golgi apparatus tethering. J. Exp. Bot. 68, 3339–3350 (2017).
Gillingham, A. K. & Munro, S. Finding the Golgi: golgin coiled-coil proteins show the way. Trends Cell Biol. 26, 399–408 (2016).
Masone, M. C., Morra, V. & Venditti, R. Illuminating the membrane contact sites between the endoplasmic reticulum and the trans-Golgi network. FEBS Lett. 593, 3135–3148 (2019).
Wong, M. & Munro, S. Membrane trafficking. The specificity of vesicle traffic to the Golgi is encoded in the golgin coiled-coil proteins. Science 346, 1256898 (2014).
Sasi, U. S. S., Ganapathy, S., Palayyan, S. R. & Gopal, R. K. Mitochondria associated membranes (MAMs): emerging drug targets for diabetes. Curr. Med. Chem. 27, 3362–3385 (2020).
Wu, S. et al. Hyperglycemia-driven inhibition of AMP-activated protein kinase α2 induces diabetic cardiomyopathy by promoting mitochondria-associated endoplasmic reticulum membranes in vivo. Circulation 139, 1913–1936 (2019).
Ma, J. H. et al. Comparative proteomic analysis of the mitochondria-associated ER membrane (MAM) in a long-term type 2 diabetic rodent model. Sci. Rep. 7, 2062 (2017).
Malli, R. & Graier, W. F. IRE1α modulates ER and mitochondria crosstalk. Nat. Cell Biol. 21, 667–668 (2019).
Gelmetti, V. et al. PINK1 and BECN1 relocalize at mitochondria-associated membranes during mitophagy and promote ER-mitochondria tethering and autophagosome formation. Autophagy 13, 654–669 (2017).
Rieusset, J. Role of endoplasmic reticulum-mitochondria communication in type 2 diabetes. Adv. Exp. Med. Biol. 997, 171–186 (2017).
Rieusset, J. et al. Disruption of calcium transfer from ER to mitochondria links alterations of mitochondria-associated ER membrane integrity to hepatic insulin resistance. Diabetologia 59, 614–623 (2016).
Hamasaki, M. et al. Autophagosomes form at ER-mitochondria contact sites. Nature 495, 389–393 (2013).
Lee, J. E., Cathey, P. I., Wu, H., Parker, R. & Voeltz, G. K. Endoplasmic reticulum contact sites regulate the dynamics of membraneless organelles. Science 367, eaay7108 (2020).
Bi, X. et al. Endoplasmic reticulum chaperone GRP78 protects heart from ischemia/reperfusion injury through Akt activation. Circ. Res. 122, 1545–1554 (2018).
Kopp, M. C., Larburu, N., Durairaj, V., Adams, C. J. & Ali, M. M. U. UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nat. Struct. Mol. Biol. 26, 1053–1062 (2019).
Karagoz, G. E., Acosta-Alvear, D. & Walter, P. The unfolded protein response: detecting and responding to fluctuations in the protein-folding capacity of the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 11, a033886 (2019).
Hetz, C. & Papa, F. R. The unfolded protein response and cell fate control. Mol. Cell 69, 169–181 (2018).
Grey, M. J. et al. IRE1β negatively regulates IRE1α signaling in response to endoplasmic reticulum stress. J. Cell Biol. 219, e201904048 (2020).
Jurkin, J. et al. The mammalian tRNA ligase complex mediates splicing of XBP1 mRNA and controls antibody secretion in plasma cells. EMBO J. 33, 2922–2936 (2014).
Yucel, S. S. et al. The metastable XBP1u transmembrane domain defines determinants for intramembrane proteolysis by signal peptide peptidase. Cell Rep. 26, 3087–3099 (2019).
Wang, D. et al. XBP1 activation enhances MANF expression via binding to endoplasmic reticulum stress response elements within MANF promoter region in hepatitis B. Int. J. Biochem. Cell Biol. 99, 140–146 (2018).
Herranen, A. et al. Deficiency of the ER-stress-regulator MANF triggers progressive outer hair cell death and hearing loss. Cell Death Dis. 11, 1–12 (2020).
Maurel, M., Chevet, E., Tavernier, J. & Gerlo, S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 39, 245–254 (2014).
Yu, J. et al. Phosphorylation switches protein disulfide isomerase activity to maintain proteostasis and attenuate ER stress. EMBO J. 39, e103841 (2020).
Eletto, D., Eletto, D., Dersh, D., Gidalevitz, T. & Argon, Y. Protein disulfide isomerase A6 controls the decay of IRE1α signaling via disulfide-dependent association. Mol. Cell 53, 562–576 (2014).
Eletto, D., Eletto, D., Boyle, S. & Argon, Y. PDIA6 regulates insulin secretion by selectively inhibiting the RIDD activity of IRE1. FASEB J. 30, 653–665 (2016).
Sepulveda, D. et al. Interactome screening identifies the ER luminal chaperone Hsp47 as a regulator of the unfolded protein response transducer IRE1α. Mol. Cell 69, 238–252 (2018).
Wang, Q. et al. Two pools of IRE1α in cardiac and skeletal muscle cells. FASEB J. 33, 8892–8904 (2019).
Takeda, K. et al. MITOL prevents ER stress-induced apoptosis by IRE1α ubiquitylation at ER-mitochondria contact sites. EMBO J. 38, e100999 (2019).
Papaioannou, A. et al. Alterations of EDEM1 functions enhance ATF6 pro-survival signaling. FEBS J. 285, 4146–4164 (2018).
Schröder, M. & Kaufman, R. J. The mammalian unfolded protein response. Annu. Rev. Biochem. 74, 739–789 (2005).
Tam, A.B. et al. The UPR activator ATF6 responds to proteotoxic and lipotoxic stress by distinct mechanisms. Dev. Cell 46, 327–343 (2018).
Zielke, S. et al. ATF4 links ER stress with reticulophagy in glioblastoma cells. Autophagy https://doi.org/10.1080/15548627.2020.1827780 (2020).
Fusakio, M. E. et al. Transcription factor ATF4 directs basal and stress-induced gene expression in the unfolded protein response and cholesterol metabolism in the liver. Mol. Biol. Cell 27, 1536–1551 (2016).
Urra, H. et al. IRE1α governs cytoskeleton remodelling and cell migration through a direct interaction with filamin A. Nat. Cell. Biol. 20, 942–953 (2018).
Ji, C. H. et al. The N-degron pathway mediates ER-phagy. Mol. Cell 75, 1058–1072 (2019).
Ji, C. H. et al. Regulation of reticulophagy by the N-degron pathway. Autophagy 16, 373–375 (2020).
Loi, M., Raimondi, A., Morone, D. & Molinari, M. ESCRT-III-driven piecemeal micro-ER-phagy remodels the ER during recovery from ER stress. Nat. Commun. 10, 5058 (2019).
Hsieh, C.-L. et al. A novel salicylanilide derivative induces autophagy cell death in castration-resistant prostate cancer via ER stress-activated PERK signaling pathway. Mol. Cancer Therapeutics 19, 101–111 (2020).
Kouroku, Y. et al. ER stress (PERK/eIF2α phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 14, 230–239 (2007).
Oh, H. J., Lee, S. & Park, P. H. ER stress contributes to autophagy induction by adiponectin in macrophages: implication in cell survival and suppression of inflammatory response. Cytokine 127, 154959 (2020).
Ajoolabady, A. et al. Enzyme-based autophagy in anti-neoplastic management: from molecular mechanisms to clinical therapeutics. Biochim. Biophys. Acta Rev. Cancer 1874, 188366 (2020).
Li, C.-F. et al. Autophagy protects HUVECs against ER stress-mediated apoptosis under simulated microgravity. Apoptosis 24, 812–825 (2019).
Wang, Y. & Tang, M. PM2.5 induces autophagy and apoptosis through endoplasmic reticulum stress in human endothelial cells. Sci. Total. Environ. 710, 136397 (2020).
Rashid, H.-O., Yadav, R. K., Kim, H.-R. & Chae, H.-J. ER stress: autophagy induction, inhibition and selection. Autophagy 11, 1956–1977 (2015).
Ni, L. et al. β-AR blockers suppresses ER stress in cardiac hypertrophy and heart failure. PLoS ONE 6, e27294 (2011).
Ortega, A. et al. Endoplasmic reticulum stress induces different molecular structural alterations in human dilated and ischemic cardiomyopathy. PLoS ONE 9, e107635 (2014).
Duan, Q. et al. MicroRNA regulation of unfolded protein response transcription factor XBP1 in the progression of cardiac hypertrophy and heart failure in vivo. J. Transl. Med. 13, 363 (2015).
Ortega, F. B., Lavie, C. J. & Blair, S. N. Obesity and cardiovascular disease. Circ. Res. 118, 1752–1770 (2016).
Dickhout, J. G., Carlisle, R. E. & Austin, R. C. Interrelationship between cardiac hypertrophy, heart failure, and chronic kidney disease: endoplasmic reticulum stress as a mediator of pathogenesis. Circ. Res. 108, 629–642 (2011).
Wang, J., Hu, X. & Jiang, H. ER stress-induced apoptosis: a novel therapeutic target in heart failure. Int. J. Cardiol. 177, 564–565 (2014).
Yao, Y. et al. A non-canonical pathway regulates ER stress signaling and blocks ER stress-induced apoptosis and heart failure. Nat. Commun. 8, 133 (2017).
Nie, J. et al. Ranolazine prevents pressure overload-induced cardiac hypertrophy and heart failure by restoring aberrant Na(+) and Ca(2+) handling. J. Cell. Physiol. 234, 11587–11601 (2019).
Gerakis, Y., Quintero, M., Li, H. & Hetz, C. The UFMylation system in proteostasis and beyond. Trends Cell. Biol. 29, 974–986 (2019).
Okada, K.-i et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110, 705–712 (2004).
Fu, H. Y. et al. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation 122, 361–369 (2010).
Zhou, X. et al. Huoxue Qianyang decoction ameliorates cardiac remodeling in obese spontaneously hypertensive rats in association with ATF6-CHOP endoplasmic reticulum stress signaling pathway regulation. Biomed. Pharmacother. 121, 109518 (2020).
Prola, A. et al. Endoplasmic reticulum stress induces cardiac dysfunction through architectural modifications and alteration of mitochondrial function in cardiomyocytes. Cardiovasc. Res. 115, 328–342 (2019).
Xu, H. X., Cui, S. M., Zhang, Y. M. & Ren, J. Mitochondrial Ca(2+) regulation in the etiology of heart failure: physiological and pathophysiological implications. Acta Pharmacol. Sin. 41, 1301–1309 (2020).
Ren, J., Pulakat, L., Whaley-Connell, A. & Sowers, J. R. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J. Mol. Med. 88, 993–1001 (2010).
Binder, P. et al. Pak2 as a novel therapeutic target for cardioprotective endoplasmic reticulum stress response. Circ. Res. 124, 696–711 (2019).
Bahar, E., Kim, J.-Y. & Yoon, H. Chemotherapy resistance explained through endoplasmic reticulum stress-dependent signaling. Cancers 11, 338 (2019).
Liu, X. et al. Endoplasmic reticulum stress sensor protein kinase R-like endoplasmic reticulum kinase (PERK) protects against pressure overload-induced heart failure and lung remodeling. Hypertension 64, 738–744 (2014).
Lu, P. D. et al. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 23, 169–179 (2004).
Steiger, D. et al. The serine/threonine-protein kinase/endoribonuclease IRE1α protects the heart against pressure overload-induced heart failure. J. Biol. Chem. 293, 9652–9661 (2018).
Hou, Y. et al. BDE-209 induces autophagy and apoptosis via IRE1α/Akt/mTOR signaling pathway in human umbilical vein endothelial cells. Environ. Pollut. 253, 429–438 (2019).
Urra, H., Pihan, P. & Hetz, C. The UPRosome – decoding novel biological outputs of IRE1alpha function. J. Cell Sci. 133, jcs218107 (2020).
Schiattarella, G. G. et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 568, 351–356 (2019).
Lu, L. et al. Irisin attenuates myocardial ischemia/reperfusion-induced cardiac dysfunction by regulating ER-mitochondria interaction through a mitochondrial ubiquitin ligase-dependent mechanism. Clin. Transl. Med. 10, e166 (2020).
Wang, Z. V. et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 156, 1179–1192 (2014).
Han, J. & Kaufman, R. J. Physiological/pathological ramifications of transcription factors in the unfolded protein response. Genes Dev. 31, 1417–1438 (2017).
Wang, S., Bian, W., Zhen, J., Zhao, L. & Chen, W. Melatonin-mediated Pak2 activation reduces cardiomyocyte death through suppressing hypoxia reoxygenation injury-induced endoplasmic reticulum stress. J.Cardiovasc. Pharmacol. 74, 20–29 (2019).
Xiao, Y. et al. Hippo pathway deletion in adult resting cardiac fibroblasts initiates a cell state transition with spontaneous and self-sustaining fibrosis. Genes Dev. 33, 1491–1505 (2019).
Stoner, M. W., McTiernan, C. F., Scott, I. & Manning, J. R. Calreticulin expression in human cardiac myocytes induces ER stress-associated apoptosis. Physiol. Rep. 8, e14400 (2020).
Gao, J. et al. Protective effect of FBXL10 in myocardial ischemia reperfusion injury via inhibiting endoplasmic reticulum stress. Respir. Med. 161, 105852 (2020).
Li, W., Li, W., Leng, Y., Xiong, Y. & Xia, Z. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA Cell Biol. 39, 210–225 (2020).
Ren, L., Wang, Q., Chen, Y., Ma, Y. & Wang, D. Involvement of MICRORNA-133a in the protective effect of hydrogen sulfide against ischemia/reperfusion-induced endoplasmic reticulum stress and cardiomyocyte apoptosis. Pharmacology 103, 1–9 (2019).
Chang, L. et al. ZYZ-803 mitigates endoplasmic reticulum stress-related necroptosis after acute myocardial infarction through downregulating the RIP3-CaMKII signaling pathway. Oxid. Med. Cell. Longev. 2019, 6173685 (2019).
Zhu, P. et al. Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: a mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol. 16, 157–168 (2018).
Wu, H.-Y., Tomizawa, K. & Matsui, H. Calpain-calcineurin signaling in the pathogenesis of calcium-dependent disorder. Acta Medica Okayama 61, 123–137 (2007).
Lu, H. T. et al. CaMKII/calpain interaction mediates ischemia/reperfusion injury in isolated rat hearts. Cell Death Dis. 11, 388 (2020).
Cao, Y. et al. Activation of γ2-AMPK suppresses ribosome biogenesis and protects against myocardial ischemia/reperfusion injury. Circ. Res. 121, 1182–1191 (2017).
Chen, L. et al. circDLPAG4/HECTD1 mediates ischaemia/reperfusion injury in endothelial cells via ER stress. RNA Biol. 17, 240–253 (2020).
Yano, T. et al. Does p53 inhibition suppress myocardial ischemia-reperfusion injury? J. Cardiovasc. Pharmacol. Ther. 23, 350–357 (2018).
Chen, Q., Thompson, J., Hu, Y., Das, A. & Lesnefsky, E. J. Cardiac specific knockout of p53 decreases ER stress-induced mitochondrial damage. Front. Cardiovasc. Med. 6, 10 (2019).
Shen, D. et al. Sulodexide attenuates endoplasmic reticulum stress induced by myocardial ischaemia/reperfusion by activating the PI3K/Akt pathway. J. Cell Mol. Med. 23, 5063–5075 (2019).
Wang, X. et al. Crocin alleviates myocardial ischemia/reperfusion-induced endoplasmic reticulum stress via regulation of miR-34a/Sirt1/Nrf2 pathway. Shock 51, 123–130 (2019).
Vatner, D. E., Oydanich, M., Zhang, J., Babici, D. & Vatner, S. F. Secreted frizzled-related protein 2, a novel mechanism to induce myocardial ischemic protection through angiogenesis. Basic. Res. Cardiol. 115, 48 (2020).
Baird, L. et al. A homeostatic shift facilitates endoplasmic reticulum proteostasis through transcriptional integration of proteostatic stress response pathways. Mol. Cell. Biol. 37, e00439-16 (2017).
Thuerauf, D. J. et al. Sarco/endoplasmic reticulum calcium ATPase-2 expression is regulated by ATF6 during the endoplasmic reticulum stress response: intracellular signaling of calcium stress in a cardiac myocyte model system. J. Biol. Chem. 276, 48309–48317 (2001).
Martindale, J. J. et al. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ. Res. 98, 1186–1193 (2006).
Doroudgar, S., Thuerauf, D. J., Marcinko, M. C., Belmont, P. J. & Glembotski, C. C. Ischemia activates the ATF6 branch of the endoplasmic reticulum stress response. J. Biol. Chem. 284, 29735–29745 (2009).
Belmont, P. J. et al. Roles for endoplasmic reticulum-associated degradation and the novel endoplasmic reticulum stress response gene derlin-3 in the ischemic heart. Circ. Res. 106, 307–316 (2010).
Lynch, J. M. et al. A thrombospondin-dependent pathway for a protective ER stress response. Cell 149, 1257–1268 (2012).
Zhang, Q. et al. Qishen Granule alleviates endoplasmic reticulum stress-induced myocardial apoptosis through IRE-1-CRYAB pathway in myocardial ischemia. J. Ethnopharmacol. 252, 112573 (2020).
Zhang, Y., Sowers, J. R. & Ren, J. Targeting autophagy in obesity: from pathophysiology to management. Nat. Rev. Endocrinol. 14, 356–376 (2018).
Yang, L., Zhao, D., Ren, J. & Yang, J. Endoplasmic reticulum stress and protein quality control in diabetic cardiomyopathy. Biochim. Biophys. Acta 1852, 209–218 (2015).
Pei, Z. et al. Inhibition of advanced glycation endproduct (AGE) rescues against streptozotocin-induced diabetic cardiomyopathy: role of autophagy and ER stress. Toxicol. Lett. 284, 10–20 (2018).
Xia, Z., Zhang, Y. & Ren, J. Endolasmic reticulum stress and metabolic syndrome: mechanisms and therapeutic potential. Acta Neuropharmacol. 2, 33–44 (2012).
Zhang, Y., Whaley-Connell, A. T., Sowers, J. R. & Ren, J. Autophagy as an emerging target in cardiorenal metabolic disease: from pathophysiology to management. Pharmacol. Ther. 191, 1–22 (2018).
Radwan, E. et al. Inhibition of endoplasmic reticulum stress ameliorates cardiovascular injury in a rat model of metabolic syndrome. J. Mol. Cell. Cardiol. 143, 15–25 (2020).
Ceylan-Isik, A. F., Sreejayan, N. & Ren, J. Endoplasmic reticulum chaperon tauroursodeoxycholic acid alleviates obesity-induced myocardial contractile dysfunction. J. Mol. Cell Cardiol. 50, 107–116 (2011).
Turdi, S., Hu, N. & Ren, J. Tauroursodeoxycholic acid mitigates high fat diet-induced cardiomyocyte contractile and intracellular Ca2+ anomalies. PLoS ONE 8, e63615 (2013).
Xiao, T. et al. Mitochondrial stress protein HSP60 regulates ER stress-induced hepatic lipogenesis. J. Mol. Endocrinol. 64, 67–75 (2020).
Hosokawa, K. et al. Ipragliflozin ameliorates endoplasmic reticulum stress and apoptosis through preventing ectopic lipid deposition in renal tubules. Int. J. Mol. Sci. 21, 190 (2019).
Vega-Martin, E. et al. Impact of caloric restriction on AMPK and endoplasmic reticulum stress in peripheral tissues and circulating peripheral blood mononuclear cells from Zucker rats. J. Nutr. Biochem. 78, 108342 (2020).
Lopez-Domenech, S. et al. Moderate weight loss attenuates chronic endoplasmic reticulum stress and mitochondrial dysfunction in human obesity. Mol. Metab. 19, 24–33 (2019).
Cui, Y. L. et al. Cholinergic drugs ameliorate endothelial dysfunction by decreasing O-GlcNAcylation via M3 AChR-AMPK-ER stress signaling. Life Sci. 222, 1–12 (2019).
Wang, Z. H. et al. Silence of TRIB3 suppresses atherosclerosis and stabilizes plaques in diabetic ApoE-/-/LDL receptor-/- mice. Diabetes 61, 463–473 (2012).
Zhang, W. et al. Skeletal muscle TRIB3 mediates glucose toxicity in diabetes and high-fat diet-induced insulin resistance. Diabetes 65, 2380–2391 (2016).
Liu, Z. et al. Circulating interleukin-1β promotes endoplasmic reticulum stress-induced myocytes apoptosis in diabetic cardiomyopathy via interleukin-1 receptor-associated kinase-2. Cardiovas. Diabetol. 14, 125 (2015).
Nam, D. H. et al. CHOP deficiency prevents methylglyoxal-induced myocyte apoptosis and cardiac dysfunction. J. Mol. Cell. Cardiol. 85, 168–177 (2015).
Zuo, S. et al. CRTH2 promotes endoplasmic reticulum stress-induced cardiomyocyte apoptosis through m-calpain. EMBO Mol. Med. 10, e8237 (2018).
Prola, A. et al. SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation. Cell Death Differ. 24, 343–356 (2017).
Yu, H. et al. Ginsenoside Rg1 ameliorates diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress-induced apoptosis in a streptozotocin-induced diabetes rat model. J. Cell. Mol. Med. 20, 623–631 (2016).
Battson, M. L., Lee, D. M. & Gentile, C. L. Endoplasmic reticulum stress and the development of endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 312, H355–H367 (2017).
Hua, L. et al. Sphingomyelin synthase 2 promotes endothelial dysfunction by inducing endoplasmic reticulum stress. Int. J. Mol. Sci. 20, 2861 (2019).
Luchetti, F. et al. Secosterol-B affects endoplasmic reticulum structure in endothelial cells. J. Steroid Biochem. Mol. Biol. 190, 234–241 (2019).
Liu, F. et al. Omentin-1 protects against high glucose-induced endothelial dysfunction via the AMPK/PPARδ signaling pathway. Biochem. Pharmacol. 174, 113830 (2020).
Wang, F. Y. et al. Icariin protects vascular endothelial cells from oxidative stress through inhibiting endoplasmic reticulum stress. J. Integr. Med. 17, 205–212 (2019).
He, Z. et al. Simvastatin attenuates H2O2-induced endothelial cell dysfunction by reducing endoplasmic reticulum stress. Molecules 24, 1782 (2019).
Fu, Z. et al. Histone deacetylase 6 reduction promotes aortic valve calcification via an endoplasmic reticulum stress-mediated osteogenic pathway. J. Thorac. Cardiovasc. Surg. 158, 408–417 (2019).
Feng, Y. et al. Selective histone deacetylase 6 Inhibitor 23BB alleviated rhabdomyolysis-induced acute kidney injury by regulating endoplasmic reticulum stress and apoptosis. Front. Pharmacol. 9, 274 (2018).
Shi, Y. et al. Fibroblast growth factor 21 attenuates vascular calcification by alleviating endoplasmic reticulum stress mediated apoptosis in rats. Int. J. Biol. Sci. 15, 138–147 (2019).
Chang, J.-R. et al. Erythropoietin attenuates vascular calcification by inhibiting endoplasmic reticulum stress in rats with chronic kidney disease. Peptides 123, 170181 (2020).
Abe, J.-i. et al. MAGI1 as a link between endothelial activation and ER stress drives atherosclerosis. JCI Insight 4, e125570 (2019).
Ochoa, C. D., Wu, R. F. & Terada, L. S. ROS signaling and ER stress in cardiovascular disease. Mol. Asp. Med. 63, 18–29 (2018).
Liu, G. et al. Inactivation of Cys(674) in SERCA2 increases BP by inducing endoplasmic reticulum stress and soluble epoxide hydrolase. Br. J. Pharmacol. 177, 1793–1805 (2019).
Carlisle, R. E. et al. Endoplasmic reticulum stress inhibition reduces hypertension through the preservation of resistance blood vessel structure and function. J. Hypertens. 34, 1556–1569 (2016).
Liu, L. et al. Berberine improves endothelial function by inhibiting endoplasmic reticulum stress in the carotid arteries of spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 458, 796–801 (2015).
Kassan, M. et al. Endoplasmic reticulum stress is involved in cardiac damage and vascular endothelial dysfunction in hypertensive mice. Arterioscler. Thromb. Vasc. Biol. 32, 1652–1661 (2012).
Han, S. et al. Inhibition of endoplasmic reticulum stress protected DOCA-salt hypertension-induced vascular dysfunction. Vascul. Pharmacol. 113, 38–46 (2019).
Chen, C. et al. Metformin prevents vascular damage in hypertension through the AMPK/ER stress pathway. Hypertens. Res. 42, 960–969 (2019).
Soliman, E., Behairy, S. F., El-Maraghy, N. N. & Elshazly, S. M. PPAR-γ agonist, pioglitazone, reduced oxidative and endoplasmic reticulum stress associated with L-NAME-induced hypertension in rats. Life Sci. 239, 117047 (2019).
Andraweera, P. H. et al. Mechanisms linking exposure to preeclampsia in utero and the risk for cardiovascular disease. J. Dev. Orig. Health Dis. 19, 1–8 (2020).
Yang, Y. et al. Endoplasmic reticulum stress may activate NLRP3. Cell Tissue Res. 379, 589–599 (2020).
Yang, M. Y. et al. Activation of aldehyde dehydrogenase 2 slows down the progression of atherosclerosis via attenuation of ER stress and apoptosis in smooth muscle cells. Acta Pharmacol. Sin. 39, 48–58 (2018).
Myoishi, M. et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation 116, 1226–1233 (2007).
Liao, X. et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 15, 545–553 (2012).
Zahid, M. D. K. et al. CCAAT/enhancer-binding protein beta (C/EBPβ) knockdown reduces inflammation, ER stress, and apoptosis, and promotes autophagy in oxLDL-treated RAW264.7 macrophage cells. Mol. Cell. Biochem. 463, 211–223 (2020).
Liang, C. P., Han, S., Li, G., Tabas, I. & Tall, A. R. Impaired MEK signaling and SERCA expression promote ER stress and apoptosis in insulin-resistant macrophages and are reversed by exenatide treatment. Diabetes 61, 2609–2620 (2012).
Trpkovic, A. et al. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit. Rev. Clin. Lab. Sci. 52, 70–85 (2015).
Cai, Z. et al. Endoplasmic reticulum stress participates in aortic valve calcification in hypercholesterolemic animals. Arterioscler. Thromb. Vasc. Biol. 33, 2345–2354 (2013).
Zhou, Z., Chen, Y., Ni, W. & Liu, T. Upregulation of nuclear factor IA suppresses oxidized low-density lipoprotein-induced endoplasmic reticulum stress and apoptosis in human umbilical vein endothelial cells. Med. Sci. Monit. 25, 1009–1016 (2019).
Lin, H. et al. Knockdown of Herp alleviates hyperhomocysteinemia mediated atherosclerosis through the inhibition of vascular smooth muscle cell phenotype switching. Int. J. Cardiol. 269, 242–249 (2018).
Thorp, E. et al. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe-/- and Ldlr-/- mice lacking CHOP. Cell Metab. 9, 474–481 (2009).
Jiang, L. et al. Inhibition of microRNA-103 attenuates inflammation and endoplasmic reticulum stress in atherosclerosis through disrupting the PTEN-mediated MAPK signaling. J. Cell Physiol. 235, 380–393 (2020).
Zhou, H., Wang, J., Zhu, P., Hu, S. & Ren, J. Ripk3 regulates cardiac microvascular reperfusion injury: the role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration. Cell Signal. 45, 12–22 (2018).
Kropski, J. A. & Blackwell, T. S. Endoplasmic reticulum stress in the pathogenesis of fibrotic disease. J. Clin. Invest. 128, 64–73 (2018).
Zhang, W., Zhu, T., Chen, L., Luo, W. & Chao, J. MCP-1 mediates ischemia-reperfusion-induced cardiomyocyte apoptosis via MCPIP1 and CaSR. Am. J. Physiol. Heart Circ. Physiol. 318, H59–H71 (2020).
Salminen, A., Kaarniranta, K. & Kauppinen, A. ER stress activates immunosuppressive network: implications for aging and Alzheimer’s disease. J. Mol. Med. 98, 633–650 (2020).
Sullivan, G. P. et al. TRAIL receptors serve as stress-associated molecular patterns to promote ER-stress-induced inflammation. Dev. Cell 52, 714–730 (2020).
Nascimento Da Conceicao, V., Sun, Y., Zboril, E. K., De la Chapa, J. J. & Singh, B. B. Loss of Ca(2+) entry via Orai-TRPC1 induces ER stress, initiating immune activation in macrophages. J. Cell Sci. 133, jcs237610 (2019).
Pang, J. et al. Mitochondrial ALDH2 protects against lipopolysaccharide-induced myocardial contractile dysfunction by suppression of ER stress and autophagy. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 1627–1641 (2019).
Chen, Z. et al. MiR-149 attenuates endoplasmic reticulum stress-induced inflammation and apoptosis in nonalcoholic fatty liver disease by negatively targeting ATF6 pathway. Immunol. Lett. 222, 40–48 (2020).
Shen, C. H. et al. Exploring the effects of tert-butylhydroperoxide induced liver injury using proteomic approach. Toxicology 316, 61–70 (2014).
Morita, S. et al. Targeting ABL-IRE1α signaling spares ER-stressed pancreatic β cells to reverse autoimmune diabetes. Cell Metab. 25, 883–897 (2017).
Li, F. et al. Resveratrol alleviates FFA and CCl4 induced apoptosis in HepG2 cells via restoring endoplasmic reticulum stress. Oncotarget 8, 43799–43809 (2017).
Chen, H. et al. The molecular mechanisms of XBP-1 gene silencing on IRE1α-TRAF2-ASK1-JNK pathways in oral squamous cell carcinoma under endoplasmic reticulum stress. Biomed. Pharmacother. 77, 108–113 (2016).
Lam, M., Marsters, S. A., Ashkenazi, A. & Walter, P. Misfolded proteins bind and activate death receptor 5 to trigger apoptosis during unresolved endoplasmic reticulum stress. eLife 9, e52291 (2020).
Lee, Y. S., Lee, D. H., Choudry, H. A., Bartlett, D. L. & Lee, Y. J. Ferroptosis-induced endoplasmic reticulum stress: cross-talk between ferroptosis and apoptosis. Mol. Cancer Res. 16, 1073–1076 (2018).
Mandl, A., Pham, L. H., Toth, K., Zambetti, G. & Erhardt, P. Puma deletion delays cardiac dysfunction in murine heart failure models through attenuation of apoptosis clinical perspective. Circulation 124, 31–39 (2011).
Wang, J., Yang, X. & Zhang, J. Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic beta cells. Cell. Signal. 28, 1099–1104 (2016).
Ren, J. et al. FUNDC1 interacts with FBXL2 to govern mitochondrial integrity and cardiac function through an IP3R3-dependent manner in obesity. Sci. Adv. 6, eabc8561 (2020).
Cao, S. S. & Kaufman, R. J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 21, 396–413 (2014).
Mennerich, D., Kellokumpu, S. & Kietzmann, T. Hypoxia and reactive oxygen species as modulators of endoplasmic reticulum and Golgi homeostasis. Antioxid. Redox Signal. 30, 113–137 (2019).
Lei, Y. et al. Toll-like receptor 4 ablation rescues against paraquat-triggered myocardial dysfunction: role of ER stress and apoptosis. Environ. Toxicol. 32, 656–668 (2017).
Kuroda, J. et al. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl Acad. Sci. USA 107, 15565–15570 (2010).
Ceylan-Isik, A. F. et al. Cardiac overexpression of metallothionein rescues cardiac contractile dysfunction and endoplasmic reticulum stress but not autophagy in sepsis. J. Mol. Cell. Cardiol. 48, 367–378 (2010).
Wang, J. et al. PERK overexpression-mediated Nrf2/HO-1 pathway alleviates hypoxia/reoxygenation-induced injury in neonatal murine cardiomyocytes via improving endoplasmic reticulum stress. Biomed. Res. Int. 2020, 6458060 (2020).
Cominacini, L. et al. Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases. Free Radic. Biol. Med. 88, 233–242 (2015).
Ajoolabady, A., Aslkhodapasandhokmabad, H., Aghanejad, A., Zhang, Y. & Ren, J. Mitophagy receptors and mediators: therapeutic targets in the management of cardiovascular ageing. Ageing Res. Rev. 62, 101129 (2020).
Bhardwaj, M., Leli, N. M., Koumenis, C. & Amaravadi, R. K. Regulation of autophagy by canonical and non-canonical ER stress responses. Semin. Cancer Biol. 66, 116–128 (2020).
Radwan, E. et al. Treg cells depletion is a mechanism that drives microvascular dysfunction in mice with established hypertension. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 403–412 (2019).
Li, Y. H. et al. Halofuginone protects against advanced glycation end products-induced injury of H9C2 cells via alleviating endoplasmic reticulum stress-associated apoptosis and inducing autophagy. Mol. Med. Rep. 20, 3131–3139 (2019).
Zhang, X. et al. Autophagy portends the level of cardiac hypertrophy in experimental hypertensive Swine model. Am. J. Hypertens. 29, 81–89 (2016).
Wiersma, M. et al. Endoplasmic reticulum stress is associated with autophagy and cardiomyocyte remodeling in experimental and human atrial fibrillation. J. Am. Heart Assoc. 6, e006458 (2017).
Wang, S. et al. Deletion of protein tyrosine phosphatase 1B obliterates endoplasmic reticulum stress-induced myocardial dysfunction through regulation of autophagy. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 3060–3074 (2017).
Gozuacik, D. et al. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 15, 1875–1886 (2008).
Han, J. et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 15, 481–490 (2013).
Castillo, K. et al. BAX inhibitor-1 regulates autophagy by controlling the IRE1α branch of the unfolded protein response. EMBO J. 30, 4465–4478 (2011).
Pires Da Silva, J. et al. SIRT1 protects the heart from ER stress-induced injury by promoting eEF2K/eEF2-dependent autophagy. Cells 9, 426 (2020).
Blackwood, E. A. et al. ATF6 regulates cardiac hypertrophy by transcriptional induction of the mTORC1 activator, Rheb. Circ. Res. 124, 79–93 (2019).
Papp, E. & Csermely, P. Chemical chaperones: mechanisms of action and potential use. Handb. Exp. Pharmacol. 172, 405–416 (2006).
Cortez, L. & Sim, V. The therapeutic potential of chemical chaperones in protein folding diseases. Prion 8, 197–202 (2014).
Engin, F. & Hotamisligil, G. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes. Metab. 12, 108–115 (2010).
Mali, V., Haddox, S., Hornersmith, C., Matrougui, K. & Belmadani, S. Essential role for EGFR tyrosine kinase and ER stress in myocardial infarction in type 2 diabetes. Pflug. Arch. 470, 471–480 (2018).
Rani, S. et al. Tauroursodeoxycholic acid (TUDCA) attenuates pressure overload-induced cardiac remodeling by reducing endoplasmic reticulum stress. PLoS ONE 12, e0176071 (2017).
Luo, H. et al. The role of tauroursodeoxycholic acid on dedifferentiation of vascular smooth muscle cells by modulation of endoplasmic reticulum stress and as an oral drug inhibiting in-stent restenosis. Cardiovasc. Drugs Ther. 33, 25–33 (2019).
Groenendyk, J. et al. Inhibition of the unfolded protein response mechanism prevents cardiac fibrosis. PLoS ONE 11, e0159682 (2016).
Bal, N. B. et al. Hypertension-induced cardiac impairment is reversed by the inhibition of endoplasmic reticulum stress. J. Pharm. Pharmacol. 71, 1809–1821 (2019).
Bal, N. B., Han, S., Kiremitci, S., Uludag, M. O. & Demirel-Yilmaz, E. Reversal of deleterious effect of hypertension on the liver by inhibition of endoplasmic reticulum stress. Mol. Biol. Rep. 47, 2243–2252 (2020).
Qin, Y. et al. Tauroursodeoxycholic acid attenuates angiotensin II induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice by inhibiting endoplasmic reticulum stress. Eur. J. Vasc. Endovasc. Surg. 53, 337–345 (2017).
Spitler, K. M., Matsumoto, T. & Webb, R. C. Suppression of endoplasmic reticulum stress improves endothelium-dependent contractile responses in aorta of the spontaneously hypertensive rat. Am. J. Physiol. Heart Circ. Physiol. 305, H344–H353 (2013).
Luo, T., Chen, B. & Wang, X. 4-PBA prevents pressure overload-induced myocardial hypertrophy and interstitial fibrosis by attenuating endoplasmic reticulum stress. Chem. Biol. Interact. 242, 99–106 (2015).
Naiel, S., Carlisle, R. E., Lu, C., Tat, V. & Dickhout, J. G. Endoplasmic reticulum stress inhibition blunts the development of essential hypertension in the spontaneously hypertensive rat. Am. J. Physiol. Heart Circ. Physiol. 316, H1214–H1223 (2019).
Jian, L., Lu, Y., Lu, S. & Lu, C. Chemical chaperone 4-phenylbutyric acid reduces cardiac ischemia/reperfusion injury by alleviating endoplasmic reticulum stress and oxidative stress. Med. Sci. Monit. 22, 5218–5227 (2016).
Luo, T. et al. Attenuation of ER stress prevents post-infarction-induced cardiac rupture and remodeling by modulating both cardiac apoptosis and fibrosis. Chem. Biol. Interact. 225, 90–98 (2015).
Bozi, L. H. M. et al. Endoplasmic reticulum stress impairs cardiomyocyte contractility through JNK-dependent upregulation of BNIP3. Int. J. Cardiol. 272, 194–201 (2018).
Huang, A. et al. 4-phenylbutyrate and valproate treatment attenuates the progression of atherosclerosis and stabilizes existing plaques. Atherosclerosis 266, 103–112 (2017).
Wang, L. et al. A novel agent enhances the chemotherapeutic efficacy of doxorubicin in MCF-7 breast cancer cells. Front. Pharmacol. 7, 249 (2016).
Wang, J. J. et al. Evaluation and treatment of endoplasmic reticulum (ER) stress in right ventricular dysfunction during monocrotaline-induced rat pulmonary arterial hypertension. Cardiovasc. Drugs Ther. 30, 587–598 (2016).
Mollazadeh, H. et al. The effect of statin therapy on endoplasmic reticulum stress. Pharmacol. Res. 137, 150–158 (2018).
Ferretti, G., Bacchetti, T. & Sahebkar, A. Effect of statin therapy on paraoxonase-1 status: a systematic review and meta-analysis of 25 clinical trials. Prog. Lipid Res. 60, 50–73 (2015).
Bahrami, A., Parsamanesh, N., Atkin, S. L., Banach, M. & Sahebkar, A. Effect of statins on toll-like receptors: a new insight to pleiotropic effects. Pharmacol. Res. 135, 230–238 (2018).
Sahebkar, A. et al. Effects of statin therapy on augmentation index as a measure of arterial stiffness: a systematic review and meta-analysis. Int. J. Cardiol. 212, 160–168 (2016).
Song, X. J. et al. Atorvastatin inhibits myocardial cell apoptosis in a rat model with post-myocardial infarction heart failure by downregulating ER stress response. Int. J. Med. Sci. 8, 564–572 (2011).
Wu, H. et al. Atorvastatin ameliorates myocardial ischemia/reperfusion injury through attenuation of endoplasmic reticulum stress-induced apoptosis. Int. J. Clin. Exp. Med. 7, 4915–4923 (2014).
Li, Y. et al. Inhibition of endoplasmic reticulum stress signaling pathway: a new mechanism of statins to suppress the development of abdominal aortic aneurysm. PLoS ONE 12, e0174821 (2017).
Hetz, C., Axten, J. M. & Patterson, J. B. Pharmacological targeting of the unfolded protein response for disease intervention. Nat. Chem. Biol. 15, 764–775 (2019).
Paxman, R. et al. Pharmacologic ATF6 activating compounds are metabolically activated to selectively modify endoplasmic reticulum proteins. eLife 7, e37168 (2018).
Grandjean, J. M. D. et al. Pharmacologic IRE1/XBP1s activation confers targeted ER proteostasis reprogramming. Nat. Chem. Biol. 16, 1052–1061 (2020).
Choy, K. W., Murugan, D. & Mustafa, M. R. Natural products targeting ER stress pathway for the treatment of cardiovascular diseases. Pharmacol. Res. 132, 119–129 (2018).
Ceylan-Isik, A. F., Fliethman, R. M., Wold, L. E. & Ren, J. Herbal and traditional Chinese medicine for the treatment of cardiovascular complications in diabetes mellitus. Curr. Diabetes Rev. 4, 320–328 (2008).
Wang, Z. G. & Ren, J. Current status and future direction of Chinese herbal medicine. Trends Pharmacol. Sci. 23, 347–348 (2002).
Bertolotti, A. The split protein phosphatase system. Biochem. J. 475, 3707–3723 (2018).
Boyce, M. et al. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307, 935–939 (2005).
Liu, C. L. et al. Salubrinal protects against tunicamycin and hypoxia induced cardiomyocyte apoptosis via the PERK-eIF2α signaling pathway. J. Geriatr. Cardiol. 9, 258–268 (2012).
Costa-Mattioli, M. & Walter, P. The integrated stress response: from mechanism to disease. Science 368, eaat5314 (2020).
Liu, Y. et al. Reduced endoplasmic reticulum stress might alter the course of heart failure via caspase-12 and JNK pathways. Can. J. Cardiol. 30, 368–375 (2014).
Li, W. et al. Blocking PERK resuces vascular smooth muscle cells from homocysteine-induced ER stress and apoptosis. Front. Biosci. 25, 536–548 (2020).
Rani, S., Sreenivasaiah, P. K., Cho, C. & Kim, D. H. Salubrinal alleviates pressure overload-induced cardiac hypertrophy by inhibiting endoplasmic reticulum stress pathway. Mol. Cell 40, 66–72 (2017).
Obafemi, T. O. et al. Metformin/donepezil combination modulates brain antioxidant status and hippocampal endoplasmic reticulum stress in type 2 diabetic rats. J. Diabetes Metab. Disord. 19, 499–510 (2020).
Liu, B., Huang, B., Liu, J. & Shi, J. S. Dendrobium nobile Lindl alkaloid and metformin ameliorate cognitive dysfunction in senescence-accelerated mice via suppression of endoplasmic reticulum stress. Brain Res. 1741, 146871 (2020).
Zeng, Z. et al. CTCF inhibits endoplasmic reticulum stress and apoptosis in cardiomyocytes by upregulating RYR2 via inhibiting S100A1. Life Sci. 242, 117158 (2020).
Liang, X., He, Q. & Zhao, Q. Effect of stains on LDL reduction and liver safety: a systematic review and meta-analysis. Biomed. Res. Int. 2018, 7092414 (2018).
Valdes, P. et al. Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc. Natl Acad. Sci. USA 111, 6804–6809 (2014).
Valencia-Sanchez, M. A., Liu, J., Hannon, G. J. & Parker, R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 20, 515–524 (2006).
Chen, M. et al. Downregulation of the miR-30 family microRNAs contributes to endoplasmic reticulum stress in cardiac muscle and vascular smooth muscle cells. Int. J. Cardiol. 173, 65–73 (2014).
Bao, Q. et al. Role of microRNA-124 in cardiomyocyte hypertrophy induced by angiotensin II. Cell. Mol. Biol. 63, 23–27 (2017).
He, L. et al. miRNA-1283 regulates the PERK/ATF4 pathway in vascular injury by targeting ATF4. PLoS ONE 11, e0159171 (2016).
Shimizu, T. et al. PERK-mediated suppression of microRNAs by sildenafil improves mitochondrial dysfunction in heart failure. iScience 23, 101410 (2020).
Bozi, L. H. et al. Aerobic exercise training rescues cardiac protein quality control and blunts endoplasmic reticulum stress in heart failure rats. J. Cell Mol. Med. 20, 2208–2212 (2016).
Chang, P. et al. Swimming exercise inhibits myocardial ER stress in the hearts of aged mice by enhancing cGMPPKG signaling. Mol. Med. Rep. 21, 549–556 (2020).
Hart, C. R. et al. Attenuated activation of the unfolded protein response following exercise in skeletal muscle of older adults. Aging 11, 7587–7604 (2019).
Bourdier, G. et al. High-intensity training reduces intermittent hypoxia-induced ER stress and myocardial infarct size. Am. J. Physiol. Heart Circ. Physiol. 310, H279–H289 (2016).
Hong, J. et al. Exercise ameliorates endoplasmic reticulum stress-mediated vascular dysfunction in mesenteric arteries in atherosclerosis. Sci. Rep. 8, 7938 (2018).
de Vicente, L. G. et al. Tlr4 participates in the responses of markers of apoptosis, inflammation, and ER stress to different acute exercise intensities in mice hearts. Life Sci. 240, 117107 (2020).
Cho, J. A. et al. Exercise and curcumin in combination improves cognitive function and attenuates ER stress in diabetic rats. Nutrients 12, 1309 (2020).
Zhu, W. et al. Endoplasmic reticulum stress may be involved in insulin resistance and lipid metabolism disorders of the white adipose tissues induced by high-fat diet containing industrial trans-fatty acids. Diabetes Metab. Syndr. Obes. 12, 1625–1638 (2019).
Blackwood, E. A. et al. Pharmacologic ATF6 activation confers global protection in widespread disease models by reprograming cellular proteostasis. Nat. Commun. 10, 187 (2019).
Noh, M. R., Kim, J. I., Han, S. J., Lee, T. J. & Park, K. M. C/EBP homologous protein (CHOP) gene deficiency attenuates renal ischemia/reperfusion injury in mice. Biochim. Biophys. Acta 1852, 1895–1901 (2015).
Mao, C. et al. Targeted mutation of the mouse Grp94 gene disrupts development and perturbs endoplasmic reticulum stress signaling. PLoS ONE 5, e10852 (2010).
Lam, C. K. et al. Novel role of HAX-1 in ischemic injury protection involvement of heat shock protein 90. Circ. Res. 112, 79–89 (2013).
Doroudgar, S. et al. Hrd1 and ER-associated protein degradation, ERAD, are critical elements of the adaptive ER stress response in cardiac myocytes. Circ. Res. 117, 536–546 (2015).
Zhong, J. et al. Therapeutic contribution of melatonin to the treatment of septic cardiomyopathy: a novel mechanism linking Ripk3-modified mitochondrial performance and endoplasmic reticulum function. Redox Biol. 26, 101287 (2019).
Shih, Y. C. et al. Endoplasmic reticulum protein TXNDC5 augments myocardial fibrosis by facilitating extracellular matrix protein folding and redox-sensitive cardiac fibroblast activation. Circ. Res. 122, 1052–1068 (2018).
Li, J. et al. Ufm1-specific ligase Ufl1 regulates endoplasmic reticulum homeostasis and protects against heart failure. Circ. Heart Fail. 11, e004917 (2018).
Yao, B. J., He, X. Q., Lin, Y. H. & Dai, W. J. Cardioprotective effects of anisodamine against myocardial ischemia/reperfusion injury through the inhibition of oxidative stress, inflammation and apoptosis. Mol. Med. Rep. 17, 1253–1260 (2018).
Xing, K. et al. Cardioprotective effect of anisodamine against myocardial ischemia injury and its influence on cardiomyocytes apoptosis. Cell Biochem. Biophys. 73, 707–716 (2015).
Shen, M. et al. Baicalin protects the cardiomyocytes from ER stress-induced apoptosis: inhibition of CHOP through induction of endothelial nitric oxide synthase. PLoS ONE 9, e88389 (2014).
Zhao, G. L. et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol. Sin. 37, 354–367 (2016).
Shu, Z. et al. Cardioprotective effects of dihydroquercetin against ischemia reperfusion injury by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt pathway. Food Funct. 10, 203–215 (2019).
Wang, M. et al. Elatoside C protects the heart from ischaemia/reperfusion injury through the modulation of oxidative stress and intracellular Ca2+ homeostasis. Int. J. Cardiol. 185, 167–176 (2015).
Guo, C. et al. Ginkgolide B ameliorates myocardial ischemia reperfusion injury in rats via inhibiting endoplasmic reticulum stress. Drug Des. Devel. Ther. 13, 767–774 (2019).
Liu, X. et al. Ginkgolide B inhibits platelet release by blocking Syk and p38 MAPK phosphorylation in thrombin-stimulated platelets. Thromb. Res. 134, 1066–1073 (2014).
Wang, S. et al. Ginkgolide K protects the heart against endoplasmic reticulum stress injury by activating the inositol-requiring enzyme 1α/X box-binding protein-1 pathway. Br. J. Pharmacol. 173, 2402–2418 (2016).
Wu, Y. et al. Icariside II prevents hypertensive heart disease by alleviating endoplasmic reticulum stress via the PERK/ATF-4/CHOP signalling pathway in spontaneously hypertensive rats. J. Pharm. Pharmacol. 71, 400–407 (2019).
Xia, Q. et al. Preparation of icariside II from icariin by enzymatic hydrolysis method. Fitoterapia 81, 437–442 (2010).
Suganya, N., Bhakkiyalakshmi, E., Suriyanarayanan, S., Paulmurugan, R. & Ramkumar, K. M. Quercetin ameliorates tunicamycin-induced endoplasmic reticulum stress in endothelial cells. Cell Prolif. 47, 231–240 (2014).
Lin, Y. et al. Inhibition of cardiomyocytes hypertrophy by resveratrol is associated with amelioration of endoplasmic reticulum stress. Cell. Physiol. Biochem. 39, 780–789 (2016).
He, Y. et al. Zn(2+) and mPTP mediate resveratrol-induced myocardial protection from endoplasmic reticulum stress. Metallomics 12, 290–300 (2020).
Lou, Y. et al. Resveratrol prevents doxorubicin-induced cardiotoxicity in H9c2 cells through the inhibition of endoplasmic reticulum stress and the activation of the Sirt1 pathway. Int. J. Mol. Med. 36, 873–880 (2015).
Guo, R. et al. SIRT1 suppresses cardiomyocyte apoptosis in diabetic cardiomyopathy: an insight into endoplasmic reticulum stress response mechanism. Int. J. Cardiol. 191, 36–45 (2015).
Shindo, S., Hosokawa, Y., Hosokawa, I., Ozaki, K. & Matsuo, T. Shikonin inhibits inflammatory cytokine production in human periodontal ligament cells. Inflammation 39, 1124–1129 (2016).
Yang, J., Wang, Z. & Chen, D. L. Shikonin ameliorates isoproterenol (ISO)-induced myocardial damage through suppressing fibrosis, inflammation, apoptosis and ER stress. Biomed. Pharmacother. 93, 1343–1357 (2017).
Li, Y. P. et al. Sulforaphane prevents rat cardiomyocytes from hypoxia/reoxygenation injury in vitro via activating SIRT1 and subsequently inhibiting ER stress. Acta Pharmacol. Sin. 37, 344–353 (2016).
Yu, Y. et al. Tournefolic acid B, derived from Clinopodium chinense (Benth.) Kuntze, protects against myocardial ischemia/reperfusion injury by inhibiting endoplasmic reticulum stress-regulated apoptosis via PI3K/AKT pathways. Phytomedicine 52, 178–186 (2019).
Acknowledgements
We express our sincere apology to those authors whose important work could not be included due to space limitations. We greatly appreciate the graphic design assistance of A. Ajoolabady (Tabriz University of Medical Sciences, Iran) and N.N. Wu (Zhongshan Hospital, Fudan University, China) for preparing the figures for initial submission. The authors’ research work was supported by the National Key R&D Program of China (2017YFA0506000), University of Wyoming Faculty Grant-in-Aid, FONDECYT 11180186, FONDAP program ANID/FONDAP/15150012, Millennium Institute P09-015-F, European Commission R&D MSCA-RISE 734749, Natural Science Foundation of China (91749128, 81770261, 81521001), Science and Technology Innovation Project of the Chinese Academy of Medical Sciences (Health and Longevity Pilot Special Project 2019-RC-HL-021) and the Training Program of Excellent Academic Leaders of Shanghai Health Mission (2018BR25).
Author information
Authors and Affiliations
Contributions
J.R. researched data for the article, contributed to discussion of the content, wrote the article and reviewed and/or edited the article before submission. Y.B., J.R.S, C.H. and Y.Z. contributed to discussion of the content and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cardiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, J., Bi, Y., Sowers, J.R. et al. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat Rev Cardiol 18, 499–521 (2021). https://doi.org/10.1038/s41569-021-00511-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-021-00511-w
This article is cited by
-
HTRA1-driven detachment of type I collagen from endoplasmic reticulum contributes to myocardial fibrosis in dilated cardiomyopathy
Journal of Translational Medicine (2024)
-
XBP1-mediated transcriptional regulation of SLC5A1 in human epithelial cells in disease conditions
Cell & Bioscience (2024)
-
Melatonin attenuates diabetic cardiomyopathy by increasing autophagy of cardiomyocytes via regulation of VEGF-B/GRP78/PERK signaling pathway
Cardiovascular Diabetology (2024)
-
O-GlcNAcylation: a pro-survival response to acute stress in the cardiovascular and central nervous systems
European Journal of Medical Research (2024)
-
Cigarette tar accelerates atherosclerosis progression via RIPK3-dependent necroptosis mediated by endoplasmic reticulum stress in vascular smooth muscle cells
Cell Communication and Signaling (2024)