Abstract
The ability to couple with multiple G protein subtypes, such as Gs, Gi/o, or Gq/11, by a given G protein-coupled receptor (GPCR) is critical for many physiological processes. Over the past few years, the cryo-EM structures for all 15 members of the medically important class B GPCRs, all in complex with Gs protein, have been determined. However, no structure of class B GPCRs with Gq/11 has been solved to date, limiting our understanding of the precise mechanisms of G protein coupling selectivity. Here we report the structures of corticotropin releasing factor receptor 2 (CRF2R) bound to Urocortin 1 (UCN1), coupled with different classes of heterotrimeric G proteins, G11 and Go. We compare these structures with the structure of CRF2R in complex with Gs to uncover the structural differences that determine the selective coupling of G protein subtypes by CRF2R. These results provide important insights into the structural basis for the ability of CRF2R to couple with multiple G protein subtypes.
Similar content being viewed by others
Introduction
G protein-coupled receptors (GPCRs) comprise a large and diverse family of cell-surface receptors, with over 800 members encoded in the human genome1. In general, GPCRs are activated by ligands and then the active GPCRs regulate diverse physiological processes through activation of heterotrimeric G proteins and other intracellular effectors2. There are four major subtypes of heterotrimeric G proteins (Gαβγ), typified by their Gα subunit: Gαs, Gαi/o, Gαq/11, and Gα12/133,4,5,6,7,8,9. Many GPCRs can couple with more than one subtype of G protein, each with a distinct coupling profile that evokes a unique cellular response, which is defined as GPCR biased activation10,11,12,13. Determining the basis for specific GPCR coupling profiles is critical to understanding their biology and pharmacology.
Corticotropin releasing factor (CRF) and three urocortin peptides (UCN1, UCN2, UCN3) are crucial stress hormones that can differentially bind to and activate CRF receptors type 1 (CRF1R) and type 2 (CRF2R), which are members of the class B GPCRs. Both CRF1R and CRF2R are thought to mediate diverse signaling pathways through their interactions with several heterotrimeric (αβγ) G protein subtypes, including different Gα subunits, such as Gαs, Gαi, Gαo, Gαq, and Gα118,9,14,15,16,17. CRF1R and CRF2R primarily activate cAMP-PKA pathways via Gs coupling, which mediate stress responses and have been implicated in the pathophysiology of various diseases14,18,19.
UCN1 is a high affinity ligand for both CRF1R and CRF2R14,20. We have previously reported cryo-EM structures of UCN1-bound CRF1R and CRF2R in complex with a heterotrimeric Gs protein21. In addition, both CRF1R and CRF2R have been shown to couple with G protein subtypes of Gq/11 and Gi/o8,14,18. Different G protein subtypes downstream of CRF1R and CRF2R were known to couple with distinct functions. For instance, the Gs-PKA signaling was reported to mediate the CRF2R function in promoting lipolysis metabolism22. In contrast, during pregnancy and labour, CRF2R couples with Gq to promote myometrial contractility and quiescence via activation of ERK and PKC pathways23,24. Moreover, coupling of CRF1R to Gi enabled Src activation and signaling of downstream Akt and ERK, which may participate in anxiety, depression and stress responsiveness25,26,27,28. However, the molecular mechanism underlying the ability of CRF2R to couple with multiple G protein subtypes remains unclear due to the lack of CRF2R structures in complex with G protein subtypes of Gq/11 and Gi/o.
In this paper, we overcome technical challenges to assemble stable complexes of UCN1-bound CRF2R with G protein subtypes of G11 and Go, and determine their cryo-EM structures. Our results provide detailed structural insights into the ability of class B GPCRs to interact with multiple G protein subtypes29.
Results
Cryo-EM structure determination of UCN1-CRF2R-G11 and UCN1-CRF2R-Go complexes
To prepare high quality human UCN1-CRF2R-G11 and UCN1-CRF2R-Go complexes, we developed the NanoBiT tethering strategy to stabilize the complexes30,31,32 (Supplementary Fig. 1a, b). We used dominant negative DNGα11 and DNGαo, which are modified forms of Gα11 and Gαo that have two amino acid replacements equivalent to those in a published dominant-negative bovine Gαs (DNGαs) construct33. In addition, DNGα11 (residues 1-24) is replaced with Gαi1 (residues 1-18) and DNGαo (residues 1-29) is replaced with Gαi1 (1-29) to possess the ability to bind scFv1634. Large-scale purification was performed to obtain the UCN1-CRF2R-G11 and UCN1-CRF2R-Go complexes for cryo-EM studies (Supplementary Fig. 1a, b).
The images of UCN1-CRF2R-G11 and UCN1-CRF2R-Go complexes were collected by a Titan Krios with a Gatan K3 detector and a Titan Krios with a Gatan K2 detector, respectively (Supplementary Figs. 2a and 3a). 2D classification showed clear secondary structure features and random distribution of the particles, which enabled a high-resolution reconstruction of the cryo-EM density maps (Supplementary Figs. 2b and 3b). The structures of the UCN1-CRF2R-G11 and UCN1-CRF2R-Go complexes were determined from a total of 3,402,020 and 1,840,659 initial particles to an overall resolution of 3.7 Å and 2.8 Å, respectively (Fig. 1, Supplementary Figs. 2c, d, 3c, d and 4a, b). Both structures were carefully examined, and refined with real space refinement in Phenix35. The model of the CRF2R-G11 complex were further refined with Rosetta refinement techniques against the cryo-EM map (Supplementary Fig. 4c–f)36. The Rosetta refinement improved the atomic details in the CRF2R-G11 complex structure that was built in a 3.7 Å cryo-EM map, with chemically optimized side chain rotamers and the global protein geometry (Supplementary Data 1). We truncated many side chains in the Rosetta-refined model of the CRF2R-G11 complex to obtain the final model for Protein Data Bank deposition, to reflect the quality of the electron density map. However, we used the side chain rotamers from the Rosetta-refined model for the purpose of discussion herein, which are indicated in the relevant figure legends.
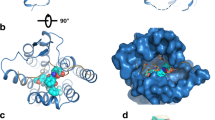
a Cartoon representation of signaling selectivity of CRF2R. b Left, cut-through view of the cryo-EM density map of the UCN1-CRF2R-Go complex and the disc-shaped micelle. The unsharpened cryo-EM density map at the 0.016 threshold shown as light gray surface indicates a micelle diameter of 11 nm. The colored cryo-EM density map is shown at the 0.022 threshold. Right, cartoon representation of the UCN1-CRF2R-Go complex is shown with annular lipids in yellow stick representation. Cornflower blue, CRF2R; coral, UCN1; dark khaki, Go; aquamarine, Gβ; deep pink, Gγ; medium purple, scFv16. c Left, cut-through view of the cryo-EM density map that represents the UCN1-CRF2R-G11 complex and the disc-shaped micelle. The unsharpened cryo-EM density map at the 0.013 threshold shown as light gray surface indicates a micelle diameter of 11 nm. The colored cryo-EM density map is shown at the 0.016 threshold. Right, cartoon representation of the UCN1-CRF2R-G11 complex shown with annular lipids in yellow stick representation.
Comparison of overall structures
The overall structures of the UCN1-CRF2R-G11 and UCN1-CRF2R-Go complexes are similar to the previous structure of the UCN1-CRF2R-Gs complex (PDB: 6PB1)21, with root mean square deviation (RMSD) values of 1.01 Å and 0.64 Å for the Cα atoms of the receptor. We observed structural differences in the second extracellular loop (ECL2), the second intracellular loop (ICL2), helix 8 (H8) of the receptor and the Gβ subunit (Fig. 2), the αN and α5 helices of Gα in these complexes (Fig. 3). Although UCN1 binds at a very similar site (Fig. 2a–c and e), the ECL2 is closer to the N terminus of UCN1 in the UCN1-CRF2R-G11 structure. The main chain carbonyl group of P3UCN1 forms an H-bond with the side-chain of K258ECL2, which was modeled with Rosetta in the UCN1-CRF2R-G11 structure and P3UCN1 also forms an H-bond with the side-chain of K258ECL2 in UCN1-CRF2R-Go structure (Fig. 2c and Supplementary Fig. 4c). Consistently, alanine mutation of K258ECL2 diminished the receptor coupling to G11 and Go compared with wild-type (WT) CRF2R, while this mutation does not affect Gs coupling of the receptor (Supplementary Fig. 5 b, e, h and Table 2). The main changes in the receptor are the different conformations of ICL2, whose conformation is critical for mediating G protein recognition and specificity (Fig. 2d), as discussed later (Fig. 4).
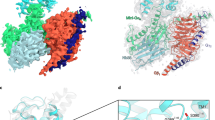
a Comparison of UCN1 and CRF2R in the G protein-bound structures in the side view. b Comparison of UCN1 and the TMD conformation in the G protein-bound structures in an extracellular view. c Comparison of the N terminus of UCN1 and the position of the ECL2 of CRF2R in the G protein-bound structures. d Comparison of the distinct conformational changes of ICL2 and the differences in the orientation of Gα in these three UCN1-CRF2R-G protein structures. e The binding pocket of UCN1(coral) in CRF2R (cornflower blue)-G11 (hot pink), UCN1(olive) in CRF2R (medium blue)-Go (dark khaki), and UCN1 (fire brick) in CRF2R (dark gray)-Gs (dark slate Blue). Many UCN1 side chains in the UCN1-CRF2R-G11 structure were truncated, whose rotamers shown here were based on the Rosetta-refined model. f Structural comparison of CRF2R H8 and Gβ1. g CRF2R H8 (cornflower blue)-Gβ1 (aquamarine) interface in the UCN1-CRF2R-G11 complex. The side chains of K372, and D379 of the receptor, and R42 and D312 of Gβ1were truncated in the structure, whose rotamers shown in this panel was from the Rosetta-refined model. h CRF2R H8 (medium blue)-Gβ1 (lime green) interface in the UCN1-CRF2R-Go complex. i CRF2R H8 (dark gray)-Gβ1 (teal) interface in the UCN1-CRF2R-Gs complex (PDB: 6PB1). The polar contacts are shown as purple dashed lines.
a Comparison of the TMD conformation and the position of the Gα-α5 helix C terminus in the G protein-bound structures is shown in cartoon representation in an intracellular view. The red arrows indicate the α5 helix of G11 and Go shift away from TM5 or ICL2, compared to those of Gs. Structure of each CRF2R-G complex was superposed onto CRF2R-G11 based on the receptor component. CRF2R (cornflower blue)-G11 (hot pink), CRF2R (medium blue)-Go (dark khaki), CRF2R (dark gray)-Gs (dark slate blue) (PDB: 6PB1). b Comparison of the TM6 conformation in three complexes. c Sequence alignment of α5 in the different Gα proteins. The red box indicate the C-tail difference of G protein. d–f Binding pocket for the Gα-α5 C terminus. d UCN1-CRF2R-Gs (PDB: 6PB1); e UCN1-CRF2R-G11; f UCN1-CRF2R-Go.The receptors are shown in cartoon and surface representations in an intracellular view. g Interactions between CRF2R and Gα-α5 in UCN1-CRF2R-Gs (PDB: 6PB1). h Interactions between CRF2R and Gα-α5 in UCN1-CRF2R-G11. Many receptor side chains and those of K345, D346, and E355 on Gα-α5 were truncated in the structure, whose rotamers shown in this panel were from the Rosetta-refined model. i Interactions between CRF2R and Gα-α5 in UCN1-CRF2R-Go. The polar contacts are shown as purple dashed lines.
a Comparison of ICL2 conformations in the UCN1-CRF2R-G11, UCN1-CRF2R-Go and UCN1-CRF2R-Gs structures (PDB: 6PB1). The red arrows indicate the relative orientation differences of the different Gα. b Sequence alignment of αN and β1 in the different Gα proteins. c Interactions between ICL2 and Gs. d Interactions between ICL2 and G11. The side chains of receptor residues, and N198, I199, and K345 of G11 in this panel were truncated in the structure. Those residues shown here were prepared based on the Rosetta-refined model. e Interactions between ICL2 and Go. The polar contacts are shown as purple dashed lines. f G protein activation and signaling assays of wild-type (WT) and ICL2 mutant CRF2R using a Gαs-Gβγ dissociation assay g using a Gα11-Gβγ dissociation assay and h using Gαo-Gβγ dissociation assay. Data from three independent experiments (n = 3) are presented as mean ± SEM.
Compared with the UCN1-CRF2R-Gs structure, H8 of CRF2R moved 2.5 Å and 2.1 Å in the opposite direction between the UCN1-CRF2R-G11 and UCN1-CRF2R-Go structures (Fig. 2f–i). The interactions between the C terminus of CRF2R and the Gβ subunit were also observed in these structures. The side chain of D3798.63b, R3768.60b and K3728.56b in H8 forms a hydrogen bond with R304, H311 and D312 in the Gβ subunit from the CRF2R-Go structure, respectively. While the side chain of R3768.60b and the main chain carbonyl of D3798.63b in H8 forms a hydrogen bond with D312 and R42 in the Gβ subunit from the CRF2R-Gs structure, respectively. In the CRF2R-G11 structure, possibly due to poor map quality, only one hydrogen bond was observed between the side chain of R3768.60b in H8 and D312 in the Gβ subunit (Fig. 2f–i, Supplementary Fig. 4d).
Besides our CRF2R-G11 complex structure, G11-coupled muscarinic acetylcholine receptor 1 (M1R) and human cytomegalovirus (HCMV) encodes GPCR US28 are the only two available G11-bound GPCR structures (PDB: 6OIJ and 7RKF)37,38. Only US28-G11 is in GDP-bound form, while both CRF2R-G11 and M1R-G11 complexes are nucleotide-free. The nucleotide-free G11 in M1R-G11 and CRF2R-G11 complexes share several common structural features, but show clear differences in conformational details between each other. To analyze how G11 couples to largely different class A M1R and class B CRF2R, we compared our CRF2R-G11 structure with the M1R-G11 complex structure. We observed that TM6 and TM7 of the two receptors adopt largely different conformations in their G11 complexes. In contrast, ICL2 of both receptors form a similar helix when binding to G11 (Fig. 5a–c), which extensively interact with the Gα-αN helix and Gα-α5 helix of the G protein. In the CRF2R-G11 structure, E220 ICL2 in ICL2 interacts weakly with R37 at the C-terminal end of the αN helix of G11, while R134ICL2 in ICL2 of M1R forms strong hydrogen bond with R37 of G11. In addition, both Y217ICL2 of the CRF2R and the related L131ICL2 of M1R interact with the β2-β3 loop and Gα-α5 helix of G11 (Fig. 5b, Supplementary Fig. 4f). We also found differences in the C termini of the receptors and the Gβ subunit in the CRF2R-G11 and M1R-G11 complexes. In the M1R-G11 complex, the C terminus after H8 is extended into a groove formed by the Ras domain of G11 and the Gβ (Fig. 5d, e)37. CRF2R had a polybasic cluster in H8 that shows different mode of interaction with Gβ (Fig. 5d, e). It seems that M1R has a polybasic C-terminal cluster to engage Gq/11 subtype of G proteins more efficiently than class B GPCRs37. In addition, there are some conformational changes of the Gα-αN and Gα-α5 helices relative to the receptor (Figs. 2d, 3 and 4), which may be important for receptor activation and transducer coupling in different Gα-bound complexes.
a Superposition of CRF2R-G11 and M1R-G11 (PDB: 6OIJ) complexes. The UCN1-CRF2R-G11 structure is colored cornflower blue (CRF2R), hot pink (G11) and aquamarine (Gα-β1); the M1R-G11 structure is colored green yellow (M1R), light coral (G11) and violet (Gα-β1). b Interaction comparison between ICL2 and G11 in CRF2R-G11 and M1R-G11 structures. Most ICL2 side chains of the receptor, the side chains of N198, I199, and K345 of Gα11 were truncated in the structure. The rotamers of those residues shown in this figure were based on the Rosetta-refined model. c Comparison of TM6, TM7 and H8-Gβ1 conformation in CRF2R-G11 and M1R-G11 complex. d Interactions comparison between CRF2R H8-Gβ1 and M1R H8-Gβ1. The side chains of K372, and D379 of the receptor, and R42 and D312 of Gβ1were truncated in the structure, whose rotamers shown in this panel was from the Rosetta-refined model. e Comparison of positively charged residues in H8 of CRF2R, at the C-terminus of M1R and electrostatic surface potential of G protein. f Comparison of the Gα11-α5-TMD interactions in the CRF2R-G11 and M1R-G11 structures. Hydrogen bonds are shown as purple dashed lines. Most side chains of the receptor, and those of K345, D346 and E355 of Gα11 were truncated in the structure. The rotamers of those residues shown in this figure were based on the Rosetta-refined model.
Comparison of the G protein-binding pockets
In the UCN1-CRF2R–G protein structures, an outward shift of TM6 at the intracellular side of the receptor forms a sharp kink, generating a common binding pocket for G protein coupling, where the C terminus of the Gα-α5 helix binds to the receptor (Fig. 3a, b). Although CRF2R shares a common binding pocket for coupling to different Gα subunits, the ability of coupling is different. The C-terminus of the α5 helix is widely considered to be the most important structural determinants of G protein coupling selectivity39,40. Comparison of these three structures shows a clear difference in the orientation of the α5 helix of G11, Go and Gs relative to CRF2R. The α5 helix of Go is rotated ~8.2° away from receptor ICL2 and the α5 helix of G11 is rotated ~2.8° towards receptor ICL2 compared to this of Gs (Fig. 4a). The sequences of the C-termini of the Gα-α5 are different among these G protein subtypes (Fig. 3c). The third and fourth to last residues are Y391 and E392 in Gs, C351 and G352 in Gi/o, and Y356 and N357 in Gq/11. These residues are less conserved among G protein subtypes and are located at the interface with TM5-6, the C-terminus of TM7 and the N-terminus of H8 of the receptor. The measured interaction interface formed between CRF2R and the α5 C terminus (residues G.H5.16 to G.H5.26) is larger for Gs (792.7 Å2) than for G11 (524.7 Å2) and Go (447.7 Å2) (Fig. 3d–f). The bulkier side chains of Y391 and E392 in Gs can form the largest interaction interface between CRF2R and the Gs-α5 C terminus (Fig. 3g–i). Therefore, class B GPCRs perform their physiological actions primarily by coupling to Gs. It can be explained by the fact that the G protein–binding pocket of class B GPCRs prefers to accommodate primarily the bulkier C-terminus of the α5 in Gs, but can still couple to the less bulkier C-terminus of the Gq/11-α5 and Gi/o-α541, consistent with the importance of the Gα C-terminus for G protein selectivity determinants39.
To accommodate the Gα-α5 helix, the cytoplasmic end of TM6 has a sharp kink as observed in all class B GPCR–G complex structures21,30,31,32,33,41,42,43,44,45,46,47,48, including the CRF2R-G11 and CRF2R-Go complexes (Fig. 3b), and the GCGR-Gi structures (PDB: 6LML)41 (Fig. 6g). The interface residues from the receptor cytoplasmic cavity that contact the C terminus of the α5 helix are highly conserved among class B GPCRs, suggesting a common mechanism of G protein coupling by class B GPCRs. Comparison of the CRF2R-G11 and CRF2R-Go structures with class A GPCR structures, including the M1R-G11 structure (PDB: 6OIJ)37, the M2R-Go structure (PDB: 6OIK)37 and the 5HT1B-mGo structure (PDB: 6G79)49, reveal different positioning of TM6 among these complexes (Figs. 5c and 6h–i). The different conformations of TM6 in these receptors allow different anchoring of the α5 helix of Gα into their distinct cytoplasmic pockets41 (Figs. 5a and 6a, b).
a–c Comparison of the TMD conformation, the position of the Gα-α5 helix C terminus and the ICL2 conformation in the Go and Gi-bound GPCR structures. The CRF2R-Go structure is colored medium blue (CRF2R) and dark khaki (Go); the M2R-Go structure is colored light sea green (M2R) and tomato (Go) (PDB: 6OIK); the GCGR-Gi structure is colored orange (GCGR) and olive drab (Gi) (PDB: 6LML); the 5HT1B-mGo structure is colored silver (5HT1B) and red (mGo) (PDB: 6G79). d Comparison of the ICL2 conformational change in the CRF2R-Go and GCGR-Gi structures. e Comparison of the ICL2 and Gα-α1 conformational change in the CRF2R-Go and M2R-Go structures. f Comparison of the ICL2 and Gα-α1 conformational change in the CRF2R-Go and 5HT1B-mGo structures. g Comparison of the Gα-α5-TM6 interactions and the H8 conformational change in the CRF2R-Go and GCGR-Gi structures. h Comparison of Gα-α5-TM6 interactions and the H8 conformation change in the CRF2R-Go and M2R-Go structures. i Comparison of Gα-α5-TM6 interactions and the H8 conformation change in the CRF2R-Go and 5HT1B-mGo structures.
Conformational differences in ICL2 determine G protein recognition and specificity
For most receptors, coupling selectivity is mainly determined by the Gα-α5 helix and the Gα subunit core39,40. In addition to the α5 helix, we also observe differences in the position of the Gα-αN and β1 strand of Gα, both elements interact with ICL2 of the receptor. Compared with the Gs-bound structure, the αN helix and β1 strand of Gα shifted away from the receptor ICL2 in the Go-bound structure and shifted towards the receptor ICL2 in the G11-bound structure (Fig. 4a). This movement are associated with the different conformations of the Gα-ICL2 interfaces of the receptor in the complexes with different G protein subtypes. The receptor ICL2 forms the most extensive interactions with Gs and the least interaction with Go, in receptor-G protein complexes (Fig. 4c–e). The distinct conformational changes of ICL2 in these three UCN1-CRF2R-G protein structures may play key roles in G protein recognition and specificity.
Comparison of the three UCN1-CRF2R-G protein structures shows clearly different conformations of ICL2 that are likely induced by the different Gα-ICL2 interfaces. ICL2 in the CRF2R-G11 and CRF2R-Gs structures adopts a short helix that forms extensive interactions with G11 and Gs (Fig. 4a, c, d). The ICL2 of the receptor in the Go complex, however, adopts a loop and forms a smaller interface with Go. While ICL2 of the receptor in Go complex is four residues shorter, the receptor TM4 is one helical turn longer than that in the complexes of Gs and G11. The extended TM4 in the CRF2R-Go structure provides additional interface that seems a compensation for the smaller G protein binding interface with the shorter loop of ICL2 (Fig. 4a, e).
In the Gs-bound structure, ICL2 forms extensive interactions with αN, the β1 strand and the α5 helix. The receptor residues T216ICL2, Y217ICL2, E220ICL2 and R2234.41b form a polar interaction network with the αN helix, the β1 strand and the α5 helix. Y217ICL2 at the middle of this helix, which is conserved in the CRF receptor family, inserts into a cavity formed by the N-terminal helix and the α5 helix of Gs (Fig. 4c). Mutagenesis of Y217ICL2 to alanine in CRF2R almost abolished coupling of Gs (Fig. 4f, Supplementary Fig. 6a, d and Table 2). Furthermore, mutations in ICL2 residues V214A3.59b, T216AICL2, S218AICL2, E220AICL2, R221AICL2, and L222AICL2 reduced Emax in Gs activation (Fig. 4f, Supplementary Fig. 6a, d and Table 2). In the G11–bound structure, T216ICL2, Y217ICL2, E220ICL2, R2234.41b formed an interface with the αN helix, the β2-β3 loop and the α5 helix of G11. Y217ICL2 formed hydrogen bonds with N198 (Fig. 4d, Supplementary Fig. 4e), mutation Y217ICL2A also abolished coupling of G11 (Fig. 4g, Supplementary Fig. 6b and Table 2). Besides Y217ICL2, mutations in ICL2 residues T216ICL2A, R2234.41bA, also reduced Emax in G11 activation. Alanine substitution of E220ICL2 showed clearly a great reduction in the potency of UCN1-mediated G11 activation, a less degree but significant reduction in the potency of UCN1-mediated Go activation and almost no effect on the potency of Gs activation (Fig.4f–h, Supplementary Fig. 6a–c and Table 2), suggesting their critical roles in G11 protein engagement and specificity. Similar interface of ICL2 with G11 are also observed in the M1R-G11 structure (Fig. 5b)37. In all these cases, the receptor ICL2 mediates G protein recognition, which may serve as a major determinant of G protein specificity.
In contrast, in CRFR-Go structure, ICL2 of CRF2R adopts an extended loop conformation, which is in a greater distance to the G protein and makes only limited contact with R24 and E28 in the αN helix of Go (Fig. 4e). Mutations of T216ICL2, Y217ICL2, S218ICL2 and R223A4.41b reduced Emax in Go activation (Fig. 4h, Supplementary Fig. 6c and Table 2). R2234.41b of the receptor has distinct role for the interaction with different G proteins. In the receptor that couples Gs or G11, R2234.41b is the second residue of a two-residue linker between ICL2 helix and TM4, and is at the interface with the G protein. In the Go-coupled receptor, however, R2234.41b is located at the second turn of TM4, with its side chain forming polar interactions with ICL2 residues T216ICL2 and S218ICL2 and stabilize the ICL2 loop conformation (Fig. 4c–e). In addition, even though Y217ICL2 does not directly interact with the hinge region of the Go protein and is in a greater distance to the Go protein than those in G11 and Gs complexes, Y217ICL2 forms many interactions with surrounding residues that stabilize the ICL2 conformation in the Go complex. The Y217 ICL2A mutation likely destabilizes the ICL2 conformation, thus indirectly affect its coupling ability to Go (Fig. 4e, h, Supplementary Fig. 6c and Table 2).
The limited contacts between ICL2 and Go in the Go-bound CRF2R structure most likely explain the lower potencies of UCN1 in stimulating Go activation and signaling when compared to those in Gs- and G11-coupled structures. A similar ICL2 loop conformation was observed in GCGR-Gi structure41, another class B GPCR that couples to Gi. The ICL2 loop of GCGR was located away from the Gi protein and its interface with Gi was weak (Fig. 6c, d). By contrast, Go-coupled structures of class A GPCRs, including M2R and 5HT1B, showed helical ICL2s closely interacted with the αN helix, β2-β3 loop and the α5 helix of Go (Fig. 6c, e, f)37,50. The above observations indicate that ICL2 is important for the G protein specificity of GPCRs.
Molecular Recognition of the α5 helices of Gs, G11 and Go
Although all three active CRF2R-G complex structures are stabilized by extensive hydrophobic and polar interactions with Gα and Gβ of the G proteins, they show different molecular details in the recognition of the interactions from receptor to α5 helices of Gs, G11 and Go (Fig. 3g–i). Comparing the recognition patterns for the α5 helix, we found that the bulky C-terminal α5 helix of Gs from Q390 to L394 forms more extensive polar and hydrophobic interactions with the receptor than G11 and Go. Specifically, Y391 in the C-terminus of the Gs α subunit, a key interface residue binds to a sub-pocket formed by R1482.46b, H1522.50b and E2053.50b, Y2083.53b, L2093.54b of CRF2R. Other interface residues are i) Gs E392, which forms polar contacts with K3106.40b and N3638.47b of the receptor; ii) Q390 of Gs, which forms a hydrogen bond with the side-chain of R1482.46b and iii) the C-terminal L394 of Gs, which forms a charge interaction with K3076.37b of the receptor TM6. In addition, Q384 and R385, at the middle of Gs-α5, forms hydrogen bond interactions with K2935.64b at the C-terminus of the receptor TM5 and S297 ICL3, respectively (Fig. 3g). Overall, the Gαs-α5 helix extensively interacts with TM2, TM3, TM5, TM6, ICL2 and ICL3, and the TM7-H8 junction of the receptor.
Compared to that in the structure of Gs-coupled CRF2R, the C-terminal α5 helix of G11 forms relatively few polar and hydrophobic contacts with the interface of CRF2R. Residues D346, L349, E355, Y356 and N357 on the α5 helix of G11 are very important for the interaction with both CRF2R and M1R (Fig. 5f, Supplementary Fig. 4e). They form large polar and hydrophobic interaction networks with the receptor. A hydrogen bond interaction is observed between CRF2R TM5 residue K2935.64b and D346 on α5 of G11, which is conserved in the interactions with Gs (corresponding residue D381) and Go (corresponding residue D341). The carboxyl group of the C-terminal V359 of G11-α5 is also observed to form a hydrogen bond with receptor residues K3076.37b and weak K3106.40b. In addition, E355 and N357 of G11-α5, forms an electrostatic interaction with R1482.46b and a hydrogen bond contact with F3627.60b, respectively (Fig. 3h, Supplementary Fig. 4e), all are important for stabilizing the interface of α5 of G11 with TM2, TM3, TM5, TM6 and TM7 of the receptor.
In contrast, the C-terminal helix of Go forms the fewest interactions with the receptor. D341 of Go-α5, corresponding to D346 of G11, forms hydrogen bonds interactions with K2935.64b. The C-terminal residue Y354 of Go shows interactions with residues K2935.64b and forms a hydrogen bond interaction with the side chain of S297ICL3 of the receptor (Fig. 3i). Mutation of S297ICL3A completely abolished UCN1 potency on Go signaling (Supplementary Figs. 5h, 6c and Table 2). C351 of Go-α5, corresponding to Y391 of Gs, has no bulky hydrophobic side chain. C351, therefore, forms only weak contacts with L2093.54b and I2133.58b on TM3 of the receptor. This is notably different to Y391 in Gs that needs a larger sub-pocket in the receptor TM bundle. L353 of Go-α5 interacts with the hydrophobic residues I2133.58b, I2865.57b, I2895.60b, L3156.45b from TM3, TM5 and TM6, respectively (Fig. 3i). These hydrophobic interactions are very important for Go activation, and their key roles in Go activation were confirmed by our mutagenesis studies. All of alanine mutations of these hydrophobic residues greatly diminished Go activation. Specially, L3156.45bA completely abolished UCN1 potency on Go signaling (Supplementary Fig. 5h, i, 6c and Table 2).
To study these important G protein subtype-specific interactions with the receptor (Supplementary Fig. 6e), we assessed UCN1-induced G protein subtype activation by serially mutated CRF2R using G protein dissociation assay and UCN1-induced cAMP accumulation assay (Supplementary Figs. 5, 6 and Table 2). Although the recognition patterns of the C-terminal α5 helix of Gα by the receptor is different, mutations of R1482.46bA, H1522.50bA, E2053.50bA, L2093.54bA, K2935.64bA, L2945.65bA, K3076.37bA and L3156.45bA, reduced UCN1 potency or Emax in any G protein activation (Supplementary Figs. 5, 6 and Table 2). These alanine mutations break the interaction networks required in any G protein activation. I2895.60bA abolished the coupling of G11 and reduced Emax in Go activation, which only slightly alters Gs activation. V3146.44bA and Y3596.45bA abolished the coupling of G11, but reduced Emax in Gs activation, which only slightly alters Go activation. On the contrary, L2905.60bA reduced efficiency and potency in Go activation, but showed significantly weaker effects on Gs and G11 activation. Receptor residue S297ICL3 is at the interface with both Gα-α5 and β3 of any coupled G protein. Its mutation to alanine decreased UCN1 potency in any G protein activation and Go proteins appeared to be the most sensitive to amino acid substitution at S297ICL3A because the C-terminal residue Y354 of Go forms a hydrogen bond with S297ICL3, which is consistent with S301ICL3 in CRF1R reported previously (Supplementary Fig. 5b, c, e, f, h, i, 6 and Table 2)8. Due to its interaction with specific G protein residues, this Ser plays a significant role in determining the G protein activation efficiency of the CRF receptors. Similar to the M1R-G11 complex (PDB: 6OIJ), most of the interactions occurring between TM5 and TM6 of CRF2R and above identified G11 residues may be critical for stabilizing the conformations of these TM segments for optimal interactions with the C terminus of the G protein (Fig. 5f).
Discussion
Here, we show the structures of CRF2R-G11 and CRF2R-Go complexes obtained by cryo-EM. Although the overall architecture of UCN1-CRF2R-G11 and UCN1-CRF2R-Go complexes are similar to that of the CRF2R-Gs complex, there are several universal and unique aspects of CRF2R coupling to different G protein subtypes. Firstly, there are clear conformational differences in ICL2 that may determine G protein recognition and specificity. Secondly, the sharp kink at the middle of TM6 facilitates formation of an open G protein–binding pocket, whose size may reflect the receptor’s ability to couple to multiple G proteins. Class B GPCRs have a large pocket at the cytoplasmic surface of the receptor that can accommodate the relatively large size of the C termini of Gα subunits, providing the basis for the predominant coupling of class B GPCRs to Gs. Class B GPCRs couple less efficiently to Gq/11 and Gi/o. The ability of class B GPCRs to coupling G protein subtypes is Gs > Gq/11 > Gi/o, which is consistent with the size of the C termini of Gα subunits, Gs > Gq/11 > Gi/o. Therefore, the CRF receptors mainly couple to Gs to mediate the cAMP-PKA pathway. It can also couple to Gq/11 to signal through the phospholipase C (PLC) pathway and exert multiple physiological actions9,51. For CRF2R, these signaling pathways regulate stress responses, blood pressure, food intake, and gastric emptying7. Although there are studies that implicate Go coupling of CRF2R8, its physiological relevance remains unclear. However, it is possible that weak coupling interactions have physiological significance under certain circumstances39. Our structures and functional assays provide critical insights into the molecular mechanisms of class B GPCR activation through multiple G protein coupling and biased agonism through selective coupling of G protein subtypes.
Methods
Constructs of CRF2R and distinct classes of heterotrimeric G proteins
The human CRF2R (residues 2-388) was cloned into pFastBac vector. The native signal peptide was replaced with the haemagglutinin signal peptide (HA). To facilitate expression and purification, the LgBiT subunit (Promega) was fused via a 15 amino acid (GSSGGGGSGGGGSSG) linker (15aa) at the C terminus, followed by a TEV protease cleavage site and a double MBP (2MBP) tag to facilitate expression and purification. A dominant-negative human Gα11 (DNGα11) and Gαo (DNGαo) construct were generated based on the published DNGαs33. Both DNGα11 and DNGαo are chimera and the N termini of DNGα11 and DNGαo were replaced with the N-terminus of Gαi1, which can bind to scFv1634. In addition, we replaced the α-helical domain of Gαo (residues 59-175) with that of Gαi1 (residues 59-174), which can bind Fab_G50 and introduced five mutations to create an Nb35 binding site. To facilitate the folding of the G protein, DNGα11 was coexpressed with GST-Ric-8A (gift from Dr. B. Kobilka) and DNGαo was coexpressed with GST-Ric-8B52. Rat Gβ1 with an N-terminal MHHHHHHSSGLVPRGSHMASHHHHHHHHHH-tag (His16) was fused with a SmBiT subunit (peptide 86, Promega)53 via a 15 amino acid GSSGGGGSGGGGSSG linker at its C terminus.
In addition, to clone the constructs into the pBiT vector (Promega) for NanoBiT assays, constructs all contained an N-terminal FLAG tag (DYKDDDD) preceded by an HA signal sequence, and were cloned into the pcDNA3.1 vector (Invitrogen) for functional studies. All constructs were cloned using homologous recombination (Clone Express One Step Cloning Kit, Vazyme Biotech) and the primers were designed for site-direct mutagenesis studies (Supplementary Table 3).
Expression and purification of CRF2R-G11 and CRF2R-Go complexes
The CRF2R and G proteins were coexpressed in Sf9 insect cells (Invitrogen). When the cells grew to a density of 3.0 × 106 cells per mL in ESF 921 cell culture medium (Expression Systems), we infected the cells with six separate virus preparations at a ratio of 1:3:3:3:3:3 for CRF2R-15aa-LgBiT-2MBP, DNG11 or DNGo, His16-Gβ1-peptide 86, Gγ2, scFv16 and GST-Ric-8A or GST-Ric-8B. The infected cells were cultured at 27 °C for 48 h before collection by centrifugation and the cell pellets were stored at −80 °C.
It was resuspended in 20 mM HEPES pH 7.4, 100 mM NaCl, 10 mM MgCl2, 10 mM CaCl2, 2 mM MnCl2, 10% glycerol, 0.1 mM TCEP, 25 mU/mL apyrase (Sigma), 10 µM UCN1(Synpeptide Co., Ltd),supplemented with Protease Inhibitor Cocktail (TargetMol, 1 mL/100 mL suspension). The lysate was incubated for 1 h at room temperature and complex from membranes solubilized by 0.5% (w/v) lauryl maltose neopentylglycol (LMNG, Anatrace) supplemented with 0.1% (w/v) cholesteryl hemisuccinate TRIS salt (CHS, Anatrace) for 2 h at 4 °C. The supernatant was isolated by centrifugation at 65,000 × g for 40 min, and the solubilized complex was incubated with Amylose resin (NEB) for 2 h at 4 °C. The resin was loaded onto a plastic gravity flow column and washed with 15 column volumes of 20 mM HEPES, pH 7.4, 100 mM NaCl,10% glycerol,10 mM MgCl2, 1 mM MnCl2, 0.01% (w/v) LMNG, 0.01% glyco-diosgenin (GDN, Anatrace) and 0.004% (w/v) CHS, 2 µM UCN1, and 25 μM TCEP. After washing, the protein was treated overnight with TEV protease on column at 4 °C. Next day the flow through was collected and concentrated, then UCN1-CRF2R-Go and UCN1-CRF2R-G11 were loaded onto a Superdex200 10/300 GL column and Superose6 Increase 10/300GL (GE Healthcare), respectively, with the buffer containing 20 mM HEPES, pH 7.4, 100 mM NaCl, 2 mM MgCl2, 0.00075% (w/v) LMNG, 0.00025% GDN, 0.0002% (w/v) CHS, 2 µM UCN1, and 100 μM TCEP. The complex fractions were collected and concentrated individually for electron microscopy experiments. The final yield of the purified complex was approximately 0.5 mg per liter of insect cell culture21,33.
Cryo-EM data acquisition
For the preparation of cryo-EM grids, 2.5 μL of the purified UCN1-CRF2R-Go and UCN1-CRF2R-G11 complexes at a concentration of ~10.0 mg/ml were respectively applied to the glow-discharged Au 200 mesh and 300 mesh holey carbon grids (Quantifoil R1.2/1.3). The grids were blotted and then plunge-frozen in liquid ethane using a Vitrobot Mark IV (ThermoFisher Scientific).
Cryo-EM images of UCN1-CRF2R-Go were collected on a Titan Krios equipped with a Gatan K2 Summit direct electron detector in the Center of Cryo-EM, Zhejiang University. The microscope was operated at 300 kV accelerating voltage at a nominal magnification of 29,000 × in counting mode, corresponding to a pixel size of 1.014 Å. The total exposure time was set to 8 s with intermediate frames recorded every 0.2 s, resulting in an accumulated dose of 64 electrons per Å2. A total of 2,929 movies were collected for the UCN1-CRF2R-Go complex.
Cryo-EM images of the UCN1-CRF2R-G11 complex were collected on a Titan Krios equipped with a Gatan K3 Summit direct electron detector in Shanghai Institute of Materia Medica. The microscope was operated at 300 kV accelerating voltage, at a nominal magnification of 46,685× in counting mode, corresponding to a pixel size of 1.045 Å. In total, 4,122 movies were obtained with a defocus range of −1.2 to −2.2 μm. An accumulated dose of 80 electrons per Å2 was fractionated into a movie stack of 36 frames.
Image processing
Image stacks were subjected to beam-induced motion correction using MotionCor2.154. Contrast transfer function (CTF) parameters for each micrograph were determined by Gctf v1.0655. The data processing was further performed in RELION-3.056.
For UCN1-CRF2R-Go, auto-picking was performed by applying Laplacian-of-Gaussian blob detection and selected 1,840,659 particle projections that were subjected to reference-free 2D classification and averaging using a binned dataset with a pixel size of 2.028 Å. The subsets of 1,755,069 particle projections with well-defined averages were selected and subjected to 3D classification by employing a mask. One stable class accounting for 809,050 particles showed detailed features for all subunits and was subsequently subjected to further 3D classification with the alignment focusing on the complex. One subset showing high map quality with 171,435 particles was subject to CTF refinement, polishing, and 3D refinement. The final map has an indicated global resolution of 2.8 Å at a Fourier shell correlation (FSC) of 0.143. The local resolutions of this complex was determined using the Bsoft package (v.2.0.7) with half maps as input maps57.
For UCN1-CRF2R-G11, auto-picking was performed by applying Laplacian-of-Gaussian blob detection and selected 3,402,020 particle projections that were subjected to reference-free 2D classification and averaging using a binned dataset with a pixel size of 2.09 Å. The subsets of 2,825,032 particle projections with well-defined averages were selected and subjected to 3D classification. One good class accounting for 155,167 particles was subsequently subjected to 3D refinement. Further 3D classification with the alignment focusing on the complex by employing a mask, leading to the identification of the sub-dataset containing 94,765 particles. After last rounds of refinement, the final map has an indicated global resolution of 3.7 Å at a Fourier shell correlation (FSC) of 0.143. The local resolutions of this complex was determined using the Bsoft package (v.2.0.7) with half maps as input maps57.
Model building and refinement
The cryo-EM structure of the CRF2R-Gs-Nb35 complex (PDB code 6PB1) was used as the start for model building and refinement against the electron microscopy map. The model was docked into the electron microscopy density map using Chimera58, followed by iterative manual adjustment and rebuilding in COOT59. Real space refinement using Phenix35 were performed against the cryo-EM maps. Rosetta refinements36 was performed for the UCN1-CRF2R-G11 structure to further optimize the side chain rotamers. We used the Rosetta-refined model (including all Rosetta refined the side chains) for the purpose of discussion. We truncated many side chains in the Rosetta-refined model of the CRF2R-G11 complex as the final model for Protein Data Bank deposit to reflect the quality of the electron density map. The model statistics were validated using MolProbity60. Fitting of the refined model to the final map was analysed using model-versus-map FSC. To monitor the potential over-fitting in model building, FSCwork and FSCfree were determined by refining ‘shaken’ models against unfiltered half-map-1 and calculating the FSC of the refined models against unfiltered half-map-1 and half-map-2. The final refinement statistics were provided in Supplementary Table 2. Structural figures were prepared in Chimera and PyMOL (https://pymol.org/2/).
cAMP accumulation assay
The GloSensor cAMP assay was performed as previously described61,62. Briefly, HEK293 cells were transfected with the WT CRF2R or mutants and the GloSensor plasmid. 24 hours after transfection, cells were distributed into 96-well microplates at a density of 5 × 104 cells per well and incubated for another 24 hours at 37 °C in 5% CO2. The cells were incubated with serum-free DMEM medium containing 2% GloSensor cAMP substrate (Promega) for 2 hours at 37 °C in 5% CO2. The cells were then stimulated with increasing concentrations of UCN1. The luminescence was measured using an EnVision multi-label microplate detector (Perkin Elmer).
G protein dissociation assay
The Gα dissociation from Gβγ assay was performed as previously described63,64,65. The plasmids WT or mutated CRF2R, Gα-RLuc8 (Gαs-RLuc8, Gα11-RLuc8 or Go-RLuc8), Gβ and Gγ-GFP were transiently co-transfected into HEK293 cells. The cells were re-seeded in 96-well microplates (5 × 104 cells per well) and incubated for another 24 hours at 37 °C in 5% CO2. The cells were washed twice with HBSS (Hank’s Balanced Salt Solution) and stimulated with UCN1 at different concentrations for 2 min. The G protein dissociation signal was measured after the addition of the substrate coelenterazine 400a (5 μM) using a Mithras LB940 multimode reader (Berthold Technologies). The BRET signal was calculated as the ratio of light emission at 510 nm/400 nm.
Measurement of receptor expression by ELISA assay
HEK293 cells were transiently transfected with N-terminal Flag-tagged wild type CRF2R or mutants in 24-well plates. 48 hours after transfection, the cells were fixed with 4% (w/v) formaldehyde for 10 min followed by incubation in blocking solution (5% BSA in DPBS) for 1 hour at room temperature. The cells were incubated with anti-FLAG primary antibody (Sigma Aldrich, Cat# F1804, 1:1000) followed by incubation with secondary anti-mouse antibody (Thermo Fisher, Cat# A-21235, 1:5000) conjugated to horseradish peroxide. The tetramethyl benzidine (TMB/E) solution was added and the reaction was terminated by adding 0.25 M HCl solution. The absorbance at 450 nm was measured using the TECAN luminescence counter (Infinite M200 Pro NanoQuant) to characterize the cell surface expression level of each receptor. The expression levels of the mutants were normalized to that of the WT CRF2R. Data are shown as the mean ± SEM. Data are from three independent experiments (n = 3).
Statistical analysis
All functional data were presented as means ± standard error of the mean (S.E.M.). Statistical analysis was performed using GraphPad Prism 8.0 (GraphPad Software). Experimental data were analyzed using two-sided one-way ANOVA with Tukey’s test. P < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Cryo-EM maps generated in this study have been deposited in the Electron Microscopy Data Bank under accession codes: EMD-26103 (G11-bound CRF2R receptor), EMD-26104 (Go-bound CRF2R receptor). The atomic coordinates generated in this study have been deposited in the Protein Data Bank under accession codes: 7TRY (G11-bound CRF2R receptor) and 7TS0 (Go-bound CRF2R receptor). Due to the limitation of the map resolution, many side chains of the G11-bound CRF2R structure were truncated before PDB deposition, as compared with our Rosetta-optimized model discussed herein. The structural model with Rosetta optimized side chains is provided as a Supplementary Data 1. Source data are provided with this paper.
References
Pierce, K. L., Premont, R. T. & Lefkowitz, R. J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650 (2002).
Flock, T. et al. Selectivity determinants of GPCR-G-protein binding. Nature 545, 317–322 (2017).
Milligan, G. & Kostenis, E. Heterotrimeric G-proteins: a short history. Br. J. Pharm. 147, S46–S55 (2006).
Grammatopoulos, D. K., Randeva, H. S., Levine, M. A., Kanellopoulou, K. A. & Hillhouse, E. W. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J. Neurochem 76, 509–519 (2001).
Van Rampelbergh, J. et al. The pituitary adenylate cyclase activating polypeptide (PACAP I) and VIP (PACAP II VIP1) receptors stimulate inositol phosphate synthesis in transfected CHO cells through interaction with different G proteins. Biochim Biophys. Acta 1357, 249–255 (1997).
Schwindinger, W. F. et al. Coupling of the PTH/PTHrP receptor to multiple G-proteins. Direct demonstration of receptor activation of Gs, Gq/11, and Gi(1) by [alpha-32P]GTP-gamma-azidoanilide photoaffinity labeling. Endocrine 8, 201–209 (1998). .
Hinkle, R. T. et al. Corticotropin releasing factor 2 receptor agonists reduce the denervation-induced loss of rat skeletal muscle mass and force and increase non-atrophying skeletal muscle mass and force. J. Muscle Res. Cell Motil. 25, 539–547 (2004).
Hillhouse, E. W. & Grammatopoulos, D. K. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr. Rev. 27, 260–286 (2006).
Grammatopoulos, D. K., Randeva, H. S., Levine, M. A., Katsanou, E. S. & Hillhouse, E. W. Urocortin, but not corticotropin-releasing hormone (CRH), activates the mitogen-activated protein kinase signal transduction pathway in human pregnant myometrium: an effect mediated via R1alpha and R2beta CRH receptor subtypes and stimulation of Gq-proteins. Mol. Endocrinol. 14, 2076–2091 (2000).
Lefkowitz, R. J. A brief history of G-protein coupled receptors (Nobel Lecture). Angew. Chem. Int Ed. Engl. 52, 6366–6378 (2013).
Avet, C. et al. Effector membrane translocation biosensors reveal G protein and betaarrestin coupling profiles of 100 therapeutically relevant GPCRs. Elife 11, https://doi.org/10.7554/eLife.74101 (2022).
Hauser, A. S. et al. Common coupling map advances GPCR-G protein selectivity. Elife 11, https://doi.org/10.7554/eLife.74107 (2022).
Inoue, A. et al. Illuminating G-Protein-Coupling Selectivity of GPCRs. Cell 177, 1933–1947 (2019). e1925.
Liapakis, G., Venihaki, M., Margioris, A., Grigoriadis, D. & Gkountelias, K. Members of CRF family and their receptors: from past to future. Curr. Med Chem. 18, 2583–2600 (2011).
Brar, B. K., Chen, A., Perrin, M. H. & Vale, W. Specificity and regulation of extracellularly regulated kinase1/2 phosphorylation through corticotropin-releasing factor (CRF) receptors 1 and 2beta by the CRF/urocortin family of peptides. Endocrinology 145, 1718–1729 (2004).
Punn, A., Levine, M. A. & Grammatopoulos, D. K. Identification of signaling molecules mediating corticotropin-releasing hormone-R1alpha-mitogen-activated protein kinase (MAPK) interactions: the critical role of phosphatidylinositol 3-kinase in regulating ERK1/2 but not p38 MAPK activation. Mol. Endocrinol. 20, 3179–3195 (2006).
Papadopoulou, N. et al. Protein kinase A-induced negative regulation of the corticotropin-releasing hormone R1alpha receptor-extracellularly regulated kinase signal transduction pathway: the critical role of Ser301 for signaling switch and selectivity. Mol. Endocrinol. 18, 624–639 (2004).
Dautzenberg, F. M. & Hauger, R. L. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharm. Sci. 23, 71–77 (2002).
Grammatopoulos, D., Stirrat, G. M., Williams, S. A. & Hillhouse, E. W. The biological activity of the corticotropin-releasing hormone receptor-adenylate cyclase complex in human myometrium is reduced at the end of pregnancy. J. Clin. Endocrinol. Metab. 81, 745–751 (1996).
Yakabi, K. et al. Urocortin 1 reduces food intake and ghrelin secretion via CRF(2) receptors. Am. J. Physiol. Endocrinol. Metab. 301, E72–E82 (2011).
Ma, S. et al. Molecular Basis for Hormone Recognition and Activation of Corticotropin-Releasing Factor Receptors. Mol. Cell 77, 669–680 (2020).
Xiong, Y. et al. The local corticotropin-releasing hormone receptor 2 signalling pathway partly mediates hypoxia-induced increases in lipolysis via the cAMP-protein kinase A signalling pathway in white adipose tissue. Mol. Cell Endocrinol. 392, 106–114 (2014).
Grammatopoulos, D. K. The role of CRH receptors and their agonists in myometrial contractility and quiescence during pregnancy and labour. Front Biosci. 12, 561–571 (2007).
Petraglia, F., Imperatore, A. & Challis, J. R. Neuroendocrine mechanisms in pregnancy and parturition. Endocr. Rev. 31, 783–816 (2010).
Parra-Mercado, G. K. et al. CRF1 Receptor Signaling via the ERK1/2-MAP and Akt Kinase Cascades: Roles of Src, EGF Receptor, and PI3-Kinase Mechanisms. Front Endocrinol. 10, 869 (2019).
Hauger, R. L., Risbrough, V., Oakley, R. H., Olivares-Reyes, J. A. & Dautzenberg, F. M. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann. N. Y Acad. Sci. 1179, 120–143 (2009).
Refojo, D. et al. Corticotropin-releasing hormone activates ERK1/2 MAPK in specific brain areas. Proc. Natl Acad. Sci. USA 102, 6183–6188 (2005).
Krishnan, V. et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol. Psychiatry 64, 691–700 (2008).
Wootten, D., Miller, L. J., Koole, C., Christopoulos, A. & Sexton, P. M. Allostery and Biased Agonism at Class B G Protein-Coupled Receptors. Chem. Rev. 117, 111–138 (2017).
Duan, J. et al. Cryo-EM structure of an activated VIP1 receptor-G protein complex revealed by a NanoBiT tethering strategy. Nat. Commun. 11, 4121 (2020).
Zhou, F. et al. Structural basis for activation of the growth hormone-releasing hormone receptor. Nat. Commun. 11, 5205 (2020).
Sun, W. et al. A unique hormonal recognition feature of the human glucagon-like peptide-2 receptor. Cell Res. 30, 1098–1108 (2020).
Zhao, L. H. et al. Structure and dynamics of the active human parathyroid hormone receptor-1. Science 364, 148–153 (2019).
Maeda, S. et al. Development of an antibody fragment that stabilizes GPCR/G-protein complexes. Nat. Commun. 9, 3712 (2018).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D. Biol. Crystallogr 66, 213–221 (2010).
Wang, R. Y. et al. Automated structure refinement of macromolecular assemblies from cryo-EM maps using Rosetta. Elife 5, https://doi.org/10.7554/eLife.17219 (2016).
Maeda, S., Qu, Q., Robertson, M. J., Skiniotis, G. & Kobilka, B. K. Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science 364, 552–557 (2019).
Tsutsumi, N. et al. Atypical structural snapshots of human cytomegalovirus GPCR interactions with host G proteins. Sci. Adv. 8, eabl5442 (2022).
Okashah, N. et al. Variable G protein determinants of GPCR coupling selectivity. Proc. Natl Acad. Sci. USA 116, 12054–12059 (2019).
Conklin, B. R., Farfel, Z., Lustig, K. D., Julius, D. & Bourne, H. R. Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature 363, 274–276 (1993).
Qiao, A. et al. Structural basis of Gs and Gi recognition by the human glucagon receptor. Science 367, 1346–1352 (2020).
Liang, Y. L. et al. Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor-Gs complex. Nature 555, 121–125 (2018).
Liang, Y. L. et al. Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor. Nature 561, 492–497 (2018).
Liang, Y. L. et al. Toward a Structural Understanding of Class B GPCR Peptide Binding and Activation. Mol. Cell 77, 656–668 (2020).
Zhao, F. et al. Structural insights into hormone recognition by the human glucose-dependent insulinotropic polypeptide receptor. Elife 10, https://doi.org/10.7554/eLife.68719 (2021).
Wang, X. et al. Molecular insights into differentiated ligand recognition of the human parathyroid hormone receptor 2. Proc Natl Acad Sci U S A 118, https://doi.org/10.1073/pnas.2101279118 (2021).
Zhang, Y. et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546, 248–253 (2017).
Liang, Y. L. et al. Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature 546, 118–123 (2017).
Garcia-Nafria, J., Nehme, R., Edwards, P. C. & Tate, C. G. Cryo-EM structure of the serotonin 5-HT1B receptor coupled to heterotrimeric Go. Nature 558, 620–623 (2018).
Garcia-Nafria, J., Nehme, R., Edwards, P. C. & Tate, C. G. Cryo-EM structure of the serotonin 5-HT1B receptor coupled to heterotrimeric G(o). Nature 558, 620 (2018).
Perrin, M. H., Grace, C. R., Riek, R. & Vale, W. W. The three-dimensional structure of the N-terminal domain of corticotropin-releasing factor receptors: sushi domains and the B1 family of G protein-coupled receptors. Ann. N. Y Acad. Sci. 1070, 105–119 (2006).
Chan, P. et al. Purification of heterotrimeric G protein alpha subunits by GST-Ric-8 association: primary characterization of purified G alpha(olf). J. Biol. Chem. 286, 2625–2635 (2011).
Dixon, A. S. et al. NanoLuc Complementation Reporter Optimized for Accurate Measurement of Protein Interactions in Cells. ACS Chem. Biol. 11, 400–408 (2016).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods, https://doi.org/10.1038/nmeth.4193 (2017).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Heymann, J. B. Single particle reconstruction and validation using Bsoft for the map challenge. J. Struct. Biol. 204, 90–95 (2018).
Pettersen, E. F. et al. UCSF Chimera−a visualization system for exploratory research and analysis. J. Comput Chem. 25, 1605–1612 (2004).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr D. Biol. Crystallogr 60, 2126–2132 (2004).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D. 66, 12–21 (2010).
Hu, Q. X. et al. Constitutive Galphai coupling activity of very large G protein-coupled receptor 1 (VLGR1) and its regulation by PDZD7 protein. J. Biol. Chem. 289, 24215–24225 (2014).
Zhang, D. L. et al. Gq activity- and beta-arrestin-1 scaffolding-mediated ADGRG2/CFTR coupling are required for male fertility. Elife 7, https://doi.org/10.7554/eLife.33432 (2018).
Yang, F. et al. Structure, function and pharmacology of human itch receptor complexes. Nature 600, 164–169 (2021).
Ping, Y. Q. et al. Structures of the glucocorticoid-bound adhesion receptor GPR97-Go complex. Nature 589, 620–626 (2021).
Cheng, J. et al. Autonomous sensing of the insulin peptide by an olfactory G protein-coupled receptor modulates glucose metabolism. Cell Metab. 34, 240–255 (2022).
Acknowledgements
The cryo-EM data were collected at the Cryo-Electron Microscopy Research Center, the Shanghai Institute of Materia Medica and at the Center of Cryo-Electron Microscopy, Zhejiang University. We thanks for Z.Y. (Zhao Yang) provided the material and the new methods about the G protein dissociation. This work was supported by National Natural Science Foundation of China 32071203(L.H.Z.); the National Key R&D Program of China 2019YFA0904200 (J.P.S.); the Young Innovator Association of CAS 2018325(L.H.Z.) and SA-SIBS Scholarship Program to L.H.Z.; Ministry of Science and Technology (China) grants 2018YFA0507002 (H.E.X.), the Shanghai Municipal Science and Technology Major Project 2019SHZDZX02 (H.E.X.) and 18ZR1447800 (L.H.Z.); the CAS Strategic Priority Research Program XDB08020303 (H.E.X.); National Natural Science Foundation of China 31971195 (P.X.), 81922071 (Y.Z.) and 2100959(C.M.); Zhejiang Province Science Fund for Distinguished Young Scholars LR19H310001 (Y.Z.), Key R & D Projects of Zhejiang Province 2021C03039 (Y.Z.); Y.Z. is also supported by MOE Frontier Science Center for Brain Science & Brain-Machine Integration, Zhejiang University.
Author information
Authors and Affiliations
Contributions
L.H.Z. designed the expression constructs, purified the complexes, prepared the final samples for negative stain and data collection toward the structures, performed structure analysis, prepared figures and wrote the manuscript; L.H.Z., C.M., S.Y.J. and D.D.S. prepared the cryo-EM grids and collected cryo-EM images; L.H.Z., S.Y.J. and C.M. performed map calculations; S.Y.J., participated in figure preparation; L.H.Z. and X.E.Z. performed model building and structure analysis; X.E.Z. built and refined the structure models; X.H. performed measuring the interaction interface and the volume of bind pocket and interface; K.M. participated in manuscript writing; X.Y. designed the signaling experiment and supervised the project execution; J.P.S, P.X. and J.Y.L. conducted signaling experiments and data analysis; Y.Z. supervised C.M., D.D.S, S.Y.J, analyzed the structures, edited the manuscript. H.E.X. conceived the project, supervised L.H.Z., analyzed the data, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, LH., Lin, J., Ji, SY. et al. Structure insights into selective coupling of G protein subtypes by a class B G protein-coupled receptor. Nat Commun 13, 6670 (2022). https://doi.org/10.1038/s41467-022-33851-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33851-3
This article is cited by
-
Molecular recognition of two endogenous hormones by the human parathyroid hormone receptor-1
Acta Pharmacologica Sinica (2023)
-
Conserved class B GPCR activation by a biased intracellular agonist
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.