Subdividing Stress Groups into Eustress and Distress Groups Using Laterality Index Calculated from Brain Hemodynamic Response
Abstract
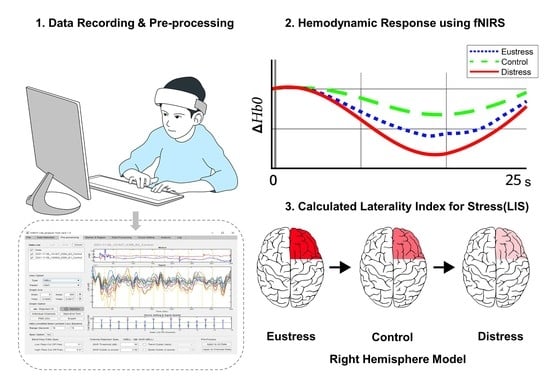
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Apparatus
2.3. Experimental Paradigm
2.4. Valence (from Negative to Positive Emotions) and Arousal (from Calm to Excitement) Ratings
2.5. Signal Processing
2.6. Anxiety and Stress Scales
2.6.1. State-Trait Anxiety Inventory (STAI)
2.6.2. Perceived Stress Scale
2.7. Physiological Data Recording: Pressure (BP) and Breath per Minute (BPM)
2.8. Behavioral Measures
2.9. Laterality Index for Stress (LIS)
2.10. Statistical Analysis
3. Results
3.1. Behavioral Results
3.2. Stress Scales and sAA
3.3. Physiological Results
3.4. Hemodynamic Responses: Two Groups
3.5. Hemodynamic Responses: Three Groups
3.6. LIS
4. Discussion
4.1. Differences in Behavioral Results
4.2. Differences in Questionnaire Results
4.3. Reduced ΔHbO Due to Stress States
4.4. Relationship between Stress and Positive or Negative Stimulation
4.5. Analysis of the LIS
4.6. Experimental Paradigm Using Target Effects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.-Y.; Stevenson, C.E.; Jung, T.-P.; Ko, L.-W. Stress-Induced Effects in Resting EEG Spectra Predict the Performance of SSVEP-Based BCI. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1771–1780. [Google Scholar] [CrossRef]
- Ragó, A.; Varga, Z.; Garami, L.; Honbolygó, F.; Csépe, V. The effect of lexical status on prosodic processing in infants learning a fixed stress language. Psychophysiology 2021, 58, e13932. [Google Scholar] [CrossRef] [PubMed]
- Vytal, K.; Cornwell, B.; Arkin, N.; Grillon, C. Describing the interplay between anxiety and cognition: From impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology 2012, 49, 842–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langlet, C.; Hainaut, J.-P.; Bolmont, B. Moderate anxiety modifies the electromyographic activity of a forearm muscle during a time-reaction task in women. Neurosci. Lett. 2017, 643, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.J.; Forman, S.D.; Franzen, P.; Berkowitz, A.; Braver, T.S.; Nystrom, L.E.; Thomas, K.M.; Noll, D.C. Sensitivity of prefrontal cortex to changes in target probability: A functional MRI study. Hum. Brain Mapp. 2001, 13, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Jodo, E.; Suzuki, Y.; Kayama, Y. Selective responsiveness of medial prefrontal cortex neurons to the meaningful stimulus with a low probability of occurrence in rats. Brain Res. 2000, 856, 68–74. [Google Scholar] [CrossRef]
- Polich, J.; Ellerson, P.C.; Cohen, J. P300, stimulus intensity, modality, and probability. Int. J. Psychophysiol. 1996, 23, 55–62. [Google Scholar] [CrossRef]
- Polich, J. Task difficulty, probability, and inter-stimulus interval as determinants of P300 from auditory stimuli. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1987, 68, 311–320. [Google Scholar] [CrossRef]
- Paulmurugan, K.; Vijayaragavan, V.; Ghosh, S.; Padmanabhan, P.; Gulyás, B. Brain–Computer Interfacing Using Functional Near-Infrared Spectroscopy (fNIRS). Biosensors 2021, 11, 389. [Google Scholar] [CrossRef]
- Ahmad Tarar, A.; Mohammad, U.; Srivastava, S.K. Wearable skin sensors and their challenges: A review of transdermal, optical, and Mechanical Sensors. Biosensors 2020, 10, 56. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, Y.; Lu, W.; Dai, X.; Tian, H.; Jiang, L. Theoretical Model for a Highly Sensitive Near Infrared Biosensor Based on Bloch Surface Wave with Dirac Semimetal. Biosensors 2021, 11, 390. [Google Scholar] [CrossRef]
- Lambert, E.; Manczak, R.; Barthout, E.; Saada, S.; Porcù, E.; Maule, F.; Bessette, B.; Viola, G.; Persano, L.; Dalmay, C.; et al. Microfluidic Lab-on-a-Chip Based on UHF-Dielectrophoresis for Stemness Phenotype Characterization and Discrimination among Glioblastoma Cells. Biosensors 2021, 11, 388. [Google Scholar] [CrossRef]
- Yeung, M.K.; Lee, T.L.; Chan, A.S. Negative mood is associated with decreased prefrontal cortex functioning during working memory in young adults. Psychophysiology 2021, 58, e13802. [Google Scholar] [CrossRef]
- Ito, H.; Yamauchi, H.; Kaneko, H.; Yoshikawa, T.; Nomura, K.; Honjo, S. Prefrontal overactivation, autonomic arousal, and task performance under evaluative pressure: A near-infrared spectroscopy (NIRS) study. Psychophysiology 2011, 48, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Kato, T.; Taneichi, K.; Matsumoto, A.; Ohtani, T.; Hamamoto, T.; Yamasue, H.; Sakano, Y.; Sasaki, T.; Sadamatsu, M.; et al. Activation of the prefrontal cortex to trauma-related stimuli measured by near-infrared spectroscopy in posttraumatic stress disorder due to terrorism. Psychophysiology 2003, 40, 492–500. [Google Scholar] [CrossRef]
- Liang, Z.; Tian, H.; Yang, H.C.; Arimitsu, T.; Takahashi, T.; Sassaroli, A.; Fantini, S.; Niu, H.; Minagawa, Y.; Tong, Y. Tracking Brain Development from Neonates to the Elderly by Hemoglobin Phase Measurement using Functional Near-infrared Spectroscopy. IEEE J. Biomed. Health Inform. 2021, 25, 2497–2509. [Google Scholar] [CrossRef]
- Selye, H. Stress without distress. In Psychopathology of Human Adaptation; Springer: Boston, MA, USA, 1976; pp. 137–146. [Google Scholar] [CrossRef]
- Anderson, C.R. Coping behaviors as intervening mechanisms in the inverted-U stress-performance relationship. J. Appl. Psychol. 1976, 61, 30. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Power, S.D. EEG-based detection of mental workload level and stress: The effect of variation in each state on classification of the other. J. Neural Eng. 2020, 17, 056015. [Google Scholar] [CrossRef] [PubMed]
- Imperatori, C.; Bersani, F.S.; Massullo, C.; Carbone, G.A.; Salvati, A.; Mazzi, G.; Cicerale, G.; Carrara, A.; Farina, B. Neurophysiological correlates of religious coping to stress: A preliminary eeg power spectra investigation. Neurosci. Lett. 2020, 728, 134956. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-T.; Cao, J.; Li, T.M. Eustress or distress: An empirical study of perceived stress in everyday college life. In Proceedings of the 2016 ACM International Joint Conference on Pervasive and Ubiquitous Computing: Adjunct, Heidelberg, Germany, 12–16 September 2016. [Google Scholar] [CrossRef]
- Kamaruddin, N. Eustress and Distress Analysis Based on Neuro-Physiological Model of Affect. Turk. J. Comput. Math. Educ. 2021, 12, 1350–1357. [Google Scholar] [CrossRef]
- Aguilar-Raab, C.; Stoffel, M.; Hernández, C.; Rahn, S.; Moessner, M.; Steinhilber, B.; Ditzen, B. Effects of a mindfulness-based intervention on mindfulness, stress, salivary alpha-amylase and cortisol in everyday life. Psychophysiology 2021, 58, e13937. [Google Scholar] [CrossRef]
- Rohleder, N.; Wolf, J.M.; Maldonado, E.F.; Kirschbaum, C. The psychosocial stress-induced increase in salivary alpha-amylase is independent of saliva flow rate. Psychophysiology 2006, 43, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.G.; Ung, W.C.; Chan, Y.L.; Lu, C.K.; Sutoko, S.; Funane, T.; Kiguchi, M.; Tang, T.B. A unified analytical framework with multiple fNIRS features for mental workload assessment in the prefrontal cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2367–2376. [Google Scholar] [CrossRef]
- Inagaki, T.K.; Eisenberger, N.I. Giving support to others reduces sympathetic nervous system-related responses to stress. Psychophysiology 2016, 53, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Seghier, M.L. Laterality index in functional MRI: Methodological issues. Magn. Reson. Imaging 2008, 26, 594–601. [Google Scholar] [CrossRef]
- Stanković, M.; Nešić, M. Functional brain asymmetry for emotions: Psychological stress-induced reversed hemispheric asymmetry in emotional face perception. Exp. Brain Res. 2020, 238, 2641–2651. [Google Scholar] [CrossRef]
- Ma, Y.; Peng, H.; Liu, H.; Gu, R.; Peng, X.; Wu, J. Alpha frontal asymmetry underlies individual differences in reactivity to acute psychosocial stress in males. Psychophysiology 2021, 58, e13893. [Google Scholar] [CrossRef]
- Lopez-Duran, N.L.; Nusslock, R.; George, C.; Kovacs, M. Frontal EEG asymmetry moderates the effects of stressful life events on internalizing symptoms in children at familial risk for depression. Psychophysiology 2012, 49, 510–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.J.B.; Urry, H.L.; Hitt, S.K.; Coan, J.A. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology 2004, 41, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Al-Shargie, F.; Tang, T.B.; Kiguchi, M. Mental stress grading based on fNIRS signals. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016. [Google Scholar] [CrossRef]
- Callister, R.; Suwarno, N.O.; Seals, D.R. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J. Physiol. 1992, 454, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Hong, J.; Kang, K.; Shin, A.; Kim, D.G.; Lee, S.; Kim, M.; Jung, I.; Kim, D. Molecular laterality encodes stress susceptibility in the medial prefrontal cortex. Mol. Brain 2021, 14, 1–17. [Google Scholar] [CrossRef]
- Wheeler, R.E.; Davidson, R.J.; Tomarken, A.J. Frontal brain asymmetry and emotional reactivity: A biological substrate of affective style. Psychophysiology 1993, 30, 82–89. [Google Scholar] [CrossRef]
- Gemignani, J.; Middell, E.; Barbour, R.L.; Graber, H.L.; Blankertz, B. Improving the analysis of near-infrared spectroscopy data with multivariate classification of hemodynamic patterns: A theoretical formulation and validation. J. Neural Eng. 2018, 15, 045001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, B.Z.; Pineda, J.A. ERPs evoked by different matrix sizes: Implications for a brain computer interface (BCI) system. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, K.; Akiyoshi, J.; Matsushita, H.; Ichioka, S.; Tanaka, Y.; Tsuru, J.; Hanada, H. Anticipatory anxiety-induced changes in human lateral prefrontal cortex activity. Biol. Psychol. 2007, 74, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. A circumplex model of affect. J. Personal. Soc. Psychol. 1980, 39, 1161. [Google Scholar] [CrossRef]
- Balconi, M.; Grippa, E.; Vanutelli, M.E. What hemodynamic (fNIRS), electrophysiological (EEG) and autonomic integrated measures can tell us about emotional processing. Brain Cogn. 2015, 95, 67–76. [Google Scholar] [CrossRef]
- Al-Shargie, F.; Kiguchi, M.; Badruddin, N.; Dass, S.C.; Hani, A.F.M.; Tang, T.B. Mental stress assessment using simultaneous measurement of EEG and fNIRS. Biomed. Opt. Express 2016, 7, 3882–3898. [Google Scholar] [CrossRef] [Green Version]
- Al-Shargie, F.; Tang, T.B.; Kiguchi, M. Stress assessment based on decision fusion of EEG and fNIRS signals. IEEE Access 2017, 5, 19889–19896. [Google Scholar] [CrossRef]
- Shin, J.; Kwon, J.; Im, C.-H. A ternary hybrid EEG-NIRS brain-computer interface for the classification of brain activation patterns during mental arithmetic, motor imagery, and idle state. Front. Neuroinform. 2018, 12, 5. [Google Scholar] [CrossRef]
- Tachtsidis, I.; Scholkmann, F. False positives and false negatives in functional near-infrared spectroscopy: Issues, challenges, and the way forward. Neurophotonic 2016, 3, 031405. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Bray, S.; Reiss, A.L. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. Neuroimage 2010, 49, 3039–3046. [Google Scholar] [CrossRef] [Green Version]
- Farahani, A.V.; Mansournia, M.A.; Asheri, H.; Fotouhi, A.; Yunesian, M.; Jamali, M.; Ziaee, V. The effects of a 10-week water aerobic exercise on the resting blood pressure in patients with essential hypertension. Asian J. Sports Med. 2010, 1, 159. [Google Scholar] [CrossRef] [Green Version]
- Tanida, M.; Katsuyama, M.; Sakatani, K. Effects of fragrance administration on stress-induced prefrontal cortex activity and sebum secretion in the facial skin. Neurosci. Lett. 2008, 432, 157–161. [Google Scholar] [CrossRef]
- Balconi, M.; Grippa, E.; Vanutelli, M.E. Resting lateralized activity predicts the cortical response and appraisal of emotions: An fNIRS study. Soc. Cogn. Affect. Neurosci. 2015, 10, 1607–1614. [Google Scholar] [CrossRef] [Green Version]
- Dunnett, C.W. Pairwise multiple comparisons in the unequal variance case. J. Am. Stat. Assoc. 1980, 75, 796–800. [Google Scholar] [CrossRef]
- Badia, S.B.; Quintero, L.V.; Cameirao, M.S.; Chirico, A.; Triberti, S.; Cipresso, P.; Gaggioli, A. Toward emotionally adaptive virtual reality for mental health applications. IEEE J. Biomed. Health Inform. 2018, 23, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Bong, C.L.; Fraser, K.; Oriot, D. Cognitive load and stress in simulation. In Comprehensive Healthcare Simulation: Pediatrics; Springer: Berlin/Heidelberg, Germany, 2016; pp. 3–17. [Google Scholar] [CrossRef]
- O’Sullivan, G. The relationship between hope, eustress, self-efficacy, and life satisfaction among undergraduates. Soc. Indic. Res. 2011, 101, 155–172. [Google Scholar] [CrossRef]
- Matthews, G.; Campbell, S.E.; Falconer, S.; Joyner, L.A.; Huggins, J.; Gilliland, K.; Rebecca, G.; Warm, J.S. Fundamental dimensions of subjective state in performance settings: Task engagement, distress, and worry. Emotion 2002, 2, 315. [Google Scholar] [CrossRef]
- Komarov, O.; Ko, L.-W.; Jung, T.-P. Associations among emotional state, sleep quality, and resting-state EEG Spectra: A longitudinal study in graduate students. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 795–804. [Google Scholar] [CrossRef]
- Reis, D.; Lehr, D.; Heber, E.; Ebert, D.D. The German version of the Perceived Stress Scale (PSS-10): Evaluation of dimensionality, validity, and measurement invariance with exploratory and confirmatory bifactor modeling. Assessment 2019, 26, 1246–1259. [Google Scholar] [CrossRef]
- Najafipour, M.; Zareizadeh, M.; Najafipour, F. Clinical and biochemical aspects between “stress” and “non-stress” induced Graves’ disease. Immunopathol. Persa 2020, 7. [Google Scholar] [CrossRef]
- Al-Shargie, F.; Tang, T.B.; Kiguchi, M. Assessment of mental stress effects on prefrontal cortical activities using canonical correlation analysis: An fNIRS-EEG study. Biomed. Opt. Express 2017, 8, 2583–2598. [Google Scholar] [CrossRef] [PubMed]
- Hinwood, M.; Morandini, J.; Day, T.A.; Walker, F.R. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb. Cortex 2012, 22, 1442–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dedovic, K.; Rexroth, M.; Wolff, E. Neural correlates of processing stressful information: An event-related fMRI study. Brain Res. 2009, 1293, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A.; Creamer, M.; O’Donnell, M.; Silove, D.; McFarlane, A.C. A multisite study of initial respiration rate and heart rate as predictors of posttraumatic stress disorder. J. Clin. Psychiatry 2008, 69, 12385. [Google Scholar] [CrossRef]
- Aeschliman, S.D.; Blue, M.S.; Williams, K.B.; Cobb, C.M.; MacNeill, S.R. A preliminary study on oxygen saturation levels of patients during periodontal surgery with and without oral conscious sedation using diazepam. J. Periodontol. 2003, 74, 1056–1059. [Google Scholar] [CrossRef]
- Casement, M.D.; Shestyuk, A.Y.; Best, J.L.; Casas, B.R.; Glezer, A.; Segundo, M.A.; Deldin, P.J. Anticipation of affect in dysthymia: Behavioral and neurophysiological indicators. Biol. Psychol. 2008, 77, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Li, J.; Suckling, J.; Feng, L. Asymmetry of hemispheric network topology reveals dissociable processes between functional and structural brain connectome in community-living elders. Front. Aging Neurosci. 2017, 9, 361. [Google Scholar] [CrossRef] [Green Version]
- Kan, R.L.; Zhang, B.B.; Zhang, J.J.; Kranz, G.S. Non-invasive brain stimulation for posttraumatic stress disorder: A systematic review and meta-analysis. Transl. Psychiatry 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Baeken, C.; De Raedt, R.; Van Schuerbeek, P.; Vanderhasselt, M.A.; De Mey, J.; Bossuyt, A.; Luypaert, R. Right prefrontal HF-rTMS attenuates right amygdala processing of negatively valenced emotional stimuli in healthy females. Behav. Brain Res. 2010, 214, 450–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocklenburg, S.; Korte, S.M.; Peterburs, J.; Wolf, O.T.; Güntürkün, O. Stress and laterality–The comparative perspective. Physiol. Behav. 2016, 164, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Reimer, B.; Song, S.; Mehler, B.; Solovey, E. Unsupervised fNIRS feature extraction with CAE and ESN autoencoder for driver cognitive load classification. J. Neural Eng. 2021, 18, 036002. [Google Scholar] [CrossRef]
- Schudlo, L.C.; Chau, T. Towards a ternary NIRS-BCI: Single-trial classification of verbal fluency task, Stroop task and unconstrained rest. J. Neural Eng. 2015, 12, 066008. [Google Scholar] [CrossRef] [PubMed]
- Nhan, B.R.; Chau, T. Classifying affective states using thermal infrared imaging of the human face. IEEE Trans. Biomed. Eng. 2009, 57, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Giannakakis, G.; Pediaditis, M.; Manousos, D.; Kazantzaki, E.; Chiarugi, F.; Simos, P.G.; Marias, K.; Tsiknakis, M. Stress and anxiety detection using facial cues from videos. Biomed. Signal Process. Control. 2017, 31, 89–101. [Google Scholar] [CrossRef]








| Two Groups (n) | p-Value ** p < 0.01 | Three Groups (n) | p-Value ** p < 0.01 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Stress | Control | Stress | |||||||
| Sub1 | Sub2 | Sub3 | Eustress | Distress | ||||||
| Number of participants | 22 | 22 | - | 11 | 11 | 11 | 11 | 11 | - | |
| Sex | Male | 15 | 15 | 1.000 | 9 | 6 | 9 | 6 | 6 | 0.329 |
| Female | 7 | 7 | 2 | 5 | 2 | 5 | 5 | |||
| Age, mean and standard deviation (SD) | 24.05 ± 2.32 | 24.41 ± 2.81 | 0.642 | 23.73 ± 1.85 | 23.09 ± 2.55 | 23.45 ± 2.46 | 24.91 ± 3.36 | 23.91 ± 2.17 | 0.773 | |
| sAA (KU/L), mean and standard deviation (SD) | 12.73 ± 9.76 | 49.82 ± 17.67 | 0.000 ** | 13.09 ± 10.90 | 13.27 ± 8.92 | 14.00 ± 11.29 | 37.82 ± 3.49 | 61.82 ± 18.07 | 0.000 ** | |
| Positive Images | Negative Images |
|---|---|
| 1340, 1463, 1710, 2045, 2058, 2071, 2158, 2216, 2340, 2347, 2352.1, 5470, 5480, 5600, 5621, 5623, 5629, 5700, 5814, 5825, 5830, 5833, 5910, 7260, 7330, 7502, 7508, 8030, 8034, 8080, 8090, 8120, 8163, 8170, 8180, 8185, 8186, 8190, 8200, 8210, 8370, 8380, 8400, 8420, 8461, 8470, 8490, 8496, 8499, 8540, | 2352.2, 2683, 2688, 2811, 3030, 3103, 3170, 3225, 3266, 3400, 3500, 3530, 3550.1, 6021, 6212, 6230, 6231, 6250.1, 6260, 6300, 6313, 6315, 6350, 6360, 6370, 6415, 6510, 6520, 6530, 6540, 6550, 6560, 6563, 6570.1, 6821, 8485, 9075, 9163, 9183, 9187, 9250, 9300, 9325, 9410, 9412, 9413, 9570, 9635.1, 9921, 9940, |
| 1410, 1440, 1441, 1460, 1540, 1604, 1610, 1630, 1920, 2035, 2040, 2151, 2156, 2165, 2306, 2314, 2331, 2332, 2341, 2388, 2391, 2395, 2550, 2598, 2650, 2660, 5000, 5001, 5200, 5201, 5202, 5210, 5220, 5551, 5594, 5611, 5631, 5725, 5760, 5779, 5780, 5781, 5811, 5829, 5831, 5836, 5891, 5982, 7325, 8497 | 2053, 2095, 2301, 2345.1, 2375.1, 2456, 2703, 2750, 2751, 2799, 2800, 2900, 3016, 3017, 3160, 3168, 3180, 3181, 3215, 3220, 3230, 3261, 3300, 3301, 3350, 6311, 6831, 9040, 9043, 9140, 9181, 9185, 9220, 9301, 9322, 9326, 9332, 9421, 9423, 9425, 9428, 9429, 9430, 9433, 9560, 9561, 9571, 9610, 9911, 9920 |
| Two Groups | sAA | Accuracy | RT: Session 1 | RT: Session 2 |
| sAA | 1 | |||
| Accuracy | 0.484 * | 1 | ||
| RT: Session 1 | 0.001 | −0.369 | 1 | |
| RT: Session 2 | −0.241 | −0.104 | 0.509 * | 1 |
| Three Groups | sAA | Accuracy | RT: Session 1 | RT: Session 2 |
| sAA | 1 | |||
| Accuracy | 0.242 | 1 | ||
| RT: Session 1 | 0.429 * | −0.347 | 1 | |
| RT: Session 2 | 0.162 | −0.405 | 0.862 ** | 1 |
| Groups | Participants (n) | Mean Score and Standard Deviation (SD) | t | p-Value * p < 0.05; ** p < 0.01 | |
|---|---|---|---|---|---|
| STAI-T | Two | Control (22) | 41.32 ± 9.27 | 2.994 | 0.005 ** |
| Stress (22) | 50.14 ± 10.24 | ||||
| Three | Control (11) | 41.32 ± 9.27 | 2.731 | 0.081 | |
| Eustress (11) | 40.82 ± 10.74 | ||||
| Distress (11) | 49.08 ± 9.80 | ||||
| STAI-S | Two | Control (22) | 39.45 ± 10.22 | 2.414 | 0.020 * |
| Stress (22) | 47.27 ± 11.24 | ||||
| Three | Control (11) | 41.32 ± 9.27 | 1.133 | 0.335 | |
| Eustress (11) | 48.00 ± 10.95 | ||||
| Distress (11) | 46.92 ± 11.52 | ||||
| PSS | Two | Control (22) | 15.91 ± 4.98 | 2.763 | 0.008 ** |
| Stress (22) | 20.68 ± 6.39 | ||||
| Three | Control (11) | 41.32 ± 9.27 | 2.469 | 0.102 | |
| Eustress (11) | 21.73 ± 5.62 | ||||
| Distress (11) | 19.25 ± 6.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bak, S.; Shin, J.; Jeong, J. Subdividing Stress Groups into Eustress and Distress Groups Using Laterality Index Calculated from Brain Hemodynamic Response. Biosensors 2022, 12, 33. https://doi.org/10.3390/bios12010033
Bak S, Shin J, Jeong J. Subdividing Stress Groups into Eustress and Distress Groups Using Laterality Index Calculated from Brain Hemodynamic Response. Biosensors. 2022; 12(1):33. https://doi.org/10.3390/bios12010033
Chicago/Turabian StyleBak, SuJin, Jaeyoung Shin, and Jichai Jeong. 2022. "Subdividing Stress Groups into Eustress and Distress Groups Using Laterality Index Calculated from Brain Hemodynamic Response" Biosensors 12, no. 1: 33. https://doi.org/10.3390/bios12010033