Selected Plant-Derived Polyphenols as Potential Therapeutic Agents for Peripheral Artery Disease: Molecular Mechanisms, Efficacy and Safety
Abstract
:1. Introduction
1.1. Mechanisms of PAD
1.2. Role of AGEs
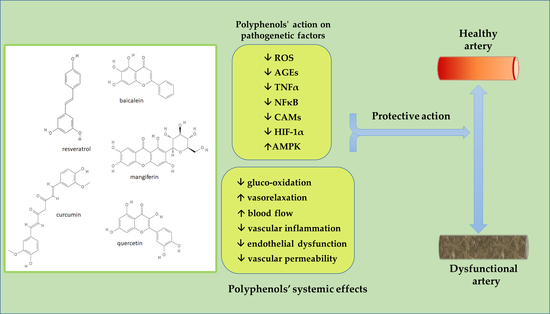
1.3. Protective Activity of Polyphenols
2. Prevention and Protective Factors: The Risk-Reduction Approach
2.1. Moderate Physical Exercise
2.2. Plant-Derived Compounds
3. Specific Polyphenolic Compounds of Interest
3.1. Baicalein
3.1.1. Chemistry and Sources
3.1.2. Activity: Preclinical Studies
3.1.3. Activity: Clinical Studies
3.1.4. Safety Profile
3.2. Curcumin
3.2.1. Chemistry and Sources
3.2.2. Activity: Preclinical Studies
3.2.3. Activity: Clinical Studies
3.2.4. Safety Profile
3.3. Mangiferin
3.3.1. Chemistry and Sources
3.3.2. Activity: Preclinical Studies
3.3.3. Activity: Clinical Studies
3.3.4. Safety Profile
3.4. Quercetin
3.4.1. Chemistry and Sources
3.4.2. Activity: Preclinical Studies
3.4.3. Activity: Clinical Studies
3.4.4. Safety Profile
3.5. Resveratrol
3.5.1. Chemistry and Sources
3.5.2. Activity: Preclinical Studies
3.5.3. Activity: Clinical Studies
3.5.4. Safety Profile
4. Conclusions
| Chemistry | Natural Compounds | Food Sources/Medicinal Plants | Experimental Models | Findings | Cellular Mechanisms | References |
|---|---|---|---|---|---|---|
| Flavones | Baicalein |
|
|
|
| [68,70,71,75,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92] |
| Diarylheptanoids | Curcumin |
|
|
|
| [100,101,106,107,108,109,110,111,112,113,114,115,122,123] |
| Xanthones | Mangiferin |
|
|
|
| [135,136,137,138,139,140,141,142,143,144,145,146,147] |
| Flavonols | Quercetin |
|
|
|
| [150,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168] |
| Stilbenes | Resveratrol |
|
|
|
| [176,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197] |
| Polyphenols | Clinical Trials | Systematic Review and Meta–Analysis | Findings | References |
|---|---|---|---|---|
| Baicalein |
| – – |
| [95,96,97] |
| Curcumin |
|
|
| [11,95,118,125,126,127,128,129] |
| Mangiferin |
| – – |
| [95,148,149] |
| Quercetin |
|
|
| [95,169,170,171,172,173,174] |
| Resveratrol |
|
|
| [44,95,199,200,201,202,203,204,205,206,207,208,209,210] |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riley, L.; Cowan, M. Non-Communicable Diseases Progress Monitor 2022; Licence: CC BY-NC-SA 3.0 IGO. Electronic Version; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-004776-1.
- Criqui, M.H.; Aboyans, V. Epidemiology of Peripheral Artery Disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, J.; Santulli, G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 2018, 275, 379–381. [Google Scholar] [CrossRef]
- Antonelli-Incalzia, R.; Pedone, C.; McDermottb, M.M.; Bandinelli, S.; Miniati, B.; Lova, R.M.; Lauretanic, F.; Ferrucci, L. Association between nutrient intake and peripheral artery disease: Results from the InCHIANTI study. Atherosclerosis 2006, 186, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Johns Hopkins University Lifetime Risk and Prevalence of Lower Extremity Peripheral Artery Disease (PAD). Available online: http://ckdpcrisk.org/padrisk/ (accessed on 28 September 2022).
- Aboyans, V.; Ricco, J.B.; Bartelink, M.L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur. J. Vasc. Endovasc. Surg. 2019, 58, S1–S109.e33. [Google Scholar] [CrossRef] [Green Version]
- Halliday, A.; Bax, J.J. The 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 301–302. [Google Scholar] [CrossRef] [Green Version]
- Brass, E.P.; Koster, D.; Hiatt, W.R.; Amato, A. A systematic review and meta-analysis of propionyl-l-carnitine effects on exercise performance in patients with claudication. Vasc. Med. 2013, 18, 3–12. [Google Scholar] [CrossRef]
- Tama, B.; Fabara, S.P.; Zarrate, D.; Anas Sohail, A. Effectiveness of Propionyl-L-Carnitine Supplementation on Exercise Performance in Intermittent Claudication: A Systematic Review. Cureus 2021, 13, e17592. [Google Scholar] [CrossRef] [PubMed]
- Kamoen, V.; Vander Stichele, R.; Campens, L.; De Bacquer, D.; Van Bortel, L.; De Backer, T. Propionyl-L-carnitine for intermittent claudication. Cochrane Database Syst. Rev. 2021, 12, CD010117. [Google Scholar] [CrossRef]
- Forster, R.; Liew, A.; Bhattacharya, V.; Shaw, J.; Stansby, G. Gene therapy for peripheral arterial disease. Cochrane Database Syst. Rev. 2018, 10(10), CD012058. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Shrivastava, S.; Cook, N.R.; Rifai, N.; Creager, M.A.; Ridker, P.M. Symptomatic peripheral arterial disease in women: Nontraditional biomarkers of elevated risk. Circulation 2008, 117, 823–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, D.; Burghaus, I.; Thiem, U.; Trampisch, U.S.; Trampisch, M.; Klaassen-Mielke, R.; Trampisch, H.-J.; Diehm, C.; Rudolf, H. The risk of peripheral artery disease in older adults-seven-year results of the getABI study. Vasa 2016, 45, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; Phillips, S.A. Assessment and prognosis of peripheral artery measures of vascular function. Prog. Cardiovasc. Dis. 2015, 57, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, 5–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lilja, E.; Bergwall, S.; Sonestedt, E.; Gottsäter, A.; Acosta, S. The association between dietary intake, lifestyle and incident symptomatic peripheral arterial disease among individuals with diabetes mellitus: Insights from the Malmö Diet and Cancer study. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819890532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimomura, M.; Fujie, S.; Sanada, K.; Kajimoto, H.; Hamaoka, T.; Iemitsu, M. Relationship between plasma asymmetric dimethylarginine and nitric oxide levels affects aerobic exercise training-induced reduction of arterial stiffness in middle-aged and older adults. Phys. Act. Nutr. 2021, 25, 16–22. [Google Scholar] [CrossRef]
- Fujie, S.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Time-dependent relationships between exercise training-induced changes in nitric oxide production and hormone regulation. Exp. Gerontol. 2022, 166, 111888. [Google Scholar] [CrossRef]
- Toma, L.; Stancu, C.S.; Sima, A.V. Endothelial dysfunction in diabetes is aggravated by glycated lipoproteins; novel molecular therapies. Biomedicines 2021, 9, 18. [Google Scholar] [CrossRef]
- Mustapha, S.; Mohammed, M.; Azemi, A.K.; Jatau, A.I.; Shehu, A.; Mustapha, L.; Aliyu, I.M.; Danraka, R.N.; Amin, A.; Bala, A.A.; et al. Current status of endoplasmic reticulum stress in type II diabetes. Molecules 2021, 26, 4362. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Pizzimenti, M.; Riou, M.; Charles, A.L.; Talha, S.; Meyer, A.; Andres, E.; Chakfé, N.; Lejay, A.; Geny, B. The rise of mitochondria in peripheral arterial disease physiopathology: Experimental and clinical data. J. Clin. Med. 2019, 8, 2125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournet, M.; Bonté, F.; Desmoulière, A. Glycation damage: A possible hub for major pathophysiological disorders and aging. Aging Dis. 2018, 9, 880–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farmer, D.G.S.; Kennedy, S. RAGE, vascular tone and vascular disease. Pharmacol. Ther. 2009, 124, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jing, J.; Yu, S.; Song, M.; Tan, H.; Cui, B.; Huang, L. Advanced glycation endproducts induce apoptosis of endothelial progenitor cells by activating receptor RAGE and NADPH oxidase/JNK signaling axis. Am. J. Transl. Res. 2016, 8, 2169–2178. [Google Scholar] [PubMed]
- Islam, R.A.; Khalsa, S.S.S.; Vyas, A.K.; Rahimian, R. Sex-specific impacts of exercise on cardiovascular remodeling. Journal of Clinical Medicine 2021, 10, 3833. [Google Scholar] [CrossRef]
- Twarda-clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Stirban, A.; Gawlowski, T.; Roden, M. Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol. Metab. 2014, 3, 94–108. [Google Scholar] [CrossRef]
- Ahmad, S.; Siddiqui, Z.; Rehman, S.; Khan, M.Y.; Khan, H.; Khanum, S.; Alouffi, S.; Saeed, M. A Glycation Angle to Look into the Diabetic Vasculopathy: Cause and Cure. Curr. Vasc. Pharmacol. 2017, 15, 352–364. [Google Scholar] [CrossRef]
- Yuan, T.; Yang, T.; Chen, H.; Fu, D.; Hu, Y.; Wang, J.; Yuan, Q.; Yu, H.; Xu, W.; Xie, X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019, 20, 247–260. [Google Scholar] [CrossRef]
- Brown, O.I.; Bridge, K.I.; Kearney, M.T. Nicotinamide adenosine dinucleotide phosphate oxidases in glucose homeostasis and diabetes-related endothelial cell dysfunction. Cells 2021, 10, 2315. [Google Scholar] [CrossRef]
- Tony Wyss, C. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 10, 180–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratyusha, S. Phenolic Compounds in the Plant Development and Defense: An Overview. In Plant Stress Physiology; Hasanuzzaman, M., Nahar, K., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, Y.; Li, C.; Zhao, J.; Wei, M.; Li, X.; Yang, S.; Mi, Y. Effect of sodium nitroprusside treatment on shikimate and phenylpropanoid pathways of apple fruit. Food Chem. 2019, 290, 263–269. [Google Scholar] [CrossRef]
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Delikanli, B. Phenolics in Human Health. Int. J. Chem. Eng. Appl. 2014, 5, 393–396. [Google Scholar] [CrossRef] [Green Version]
- Schön, C.; Allegrini, P.; Engelhart-jentzsch, K.; Riva, A.; Petrangolini, G. Grape Seed Extract PositivelyModulates Blood Pressure and Perceived Stress: ARandomized, Double-Blind, Placebo-Controlled Study in Healthy Volunteers. Nutrients 2021, 13, 654. [Google Scholar] [CrossRef]
- Saarenhovi, M.; Salo, P.; Scheinin, M.; Lehto, J.; Lovró, Z.; Tiihonen, K.; Lehtinen, M.J.; Junnila, J.; Hasselwander, O.; Tarpila, A.; et al. The effect of an apple polyphenol extract rich in epicatechin and flavan-3-ol oligomers on brachial artery flow-mediated vasodilatory function in volunteers with elevated blood pressure. Nutr. J. 2017, 16, 73. [Google Scholar] [CrossRef] [Green Version]
- Decroix, L.; Tonoli, C.; Soares, D.D.; Descat, A.; Drittij-Reijnders, M.J.; Weseler, A.R.; Bast, A.; Stahl, W.; Heyman, E.; Meeusen, R. Acute cocoa Flavanols intake has minimal effects on exercise-induced oxidative stress and nitric oxide production in healthy cyclists: A randomized controlled trial. J. Int. Soc. Sport. Nutr. 2017, 14, 28. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.; Gopi, S.; Divya, C. A Randomized Single Dose Parallel Study on Enhancement of Nitric Oxide in Serum and Saliva with the Use of Natural Sports Supplement in Healthy Adults. J. Diet. Suppl. 2018, 15, 161–172. [Google Scholar] [CrossRef]
- Nemzer, B.V.; Rodriguez, L.C.; Hammond, L.; Disilvestro, R.; Hunter, J.M.; Pietrzkowski, Z. Acute reduction of serum 8-iso-PGF2-alpha and advanced oxidation protein products in vivo by a polyphenol-rich beverage; A pilot clinical study with phytochemical and in vitro antioxidant characterization. Nutr. J. 2011, 10, 67. [Google Scholar] [CrossRef] [Green Version]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Lara, J.; Siervo, M.; Celis-Morales, C.; Mathers, J.C. Effects of exercise modalities on arterial stiffness and wave reflection: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e110034 . [Google Scholar] [CrossRef] [Green Version]
- Suganya, N.; Bhakkiyalakshmi, E.; Sarada, D.V.L.L.; Ramkumar, K.M. Reversibility of endothelial dysfunction in diabetes: Role of polyphenols. Br. J. Nutr. 2016, 116, 223–246. [Google Scholar] [CrossRef] [Green Version]
- Peñín-Grandes, S.; Martín-Hernández, J.; Valenzuela, P.L.; López-Ortiz, S.; Pinto-Fraga, J.; del Río Solá, L.; Emanuele, E.; Lista, S.; Lucia, A.; Santos-Lozano, A. Exercise and the hallmarks of peripheral arterial disease. Atherosclerosis 2022, 350, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Prior, B.M.; Lloyd, P.G.; Yang, H.T.; Terjung, R.L. Exercise-induced vascular remodeling. Exerc. Sport Sci. Rev. 2003, 31, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Ejiri, K.; Toda, H. Association between home-based exercise using a pedometer and clinical prognosis after endovascular treatment in patients with peripheral artery disease. J. Cardiol. 2022. S0914-5087(22)00228-3. [Google Scholar] [CrossRef]
- Ehrman, J.K.; Gardner, A.W.; Salisbury, D.; Lui, K.; Treat-Jacobson, D. Supervised Exercise Therapy for Symptomatic Peripheral Artery Disease: A review of current experience and practice-based recommendations. J. Cardiopulm. Rehabil. Prev. 2022, epub ahead of print. [CrossRef] [PubMed]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 69, 1465–1508. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.C.; Hart, C.R.; Layec, G.; Kwon, O.S.; Richardson, R.S.; Trinity, J.D. Impaired hemodynamic response to exercise in patients with peripheral artery disease: Evidence of a link to inflammation and oxidative stress. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2022; epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, V.; Morand, C. Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: An update for clinicians. Ther. Adv. Chronic Dis. 2012, 3, 87–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, R.P. Vascular endothelial dysfunction and nutritional compounds in early type 1 diabetes. Curr. Diabetes Rev. 2014, 10, 201–207. [Google Scholar] [CrossRef]
- Stromsnes, K.; Mas-Bargues, C.; Gambini, J.; Gimeno-Mallench, L. Protective Effects of Polyphenols Present in Mediterranean Diet on Endothelial Dysfunction. Oxidative Med. Cell. Longev. 2020, 2020, 2097096. [Google Scholar] [CrossRef]
- Oak, M.H.; Auger, C.; Belcastro, E.; Park, S.H.; Lee, H.H.; Schini-Kerth, V.B. Potential mechanisms underlying cardiovascular protection by polyphenols: Role of the endothelium. Free. Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef]
- Munir, K.M.; Chandrasekaran, S.; Gao, F.; Quon, M.J. Mechanisms for food polyphenols to ameliorate insulin resistance and endothelial dysfunction: Therapeutic implications for diabetes and its cardiovascular complications. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E679–E686. [Google Scholar] [CrossRef] [Green Version]
- Woodward, K.A.; Draijer, R.; Thijssen, D.H.J.; Low, D.A. Polyphenols and Microvascular Function in Humans: A Systematic Review. Curr. Pharm. Des. 2018, 24, 203–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donat-Vargas, C.; Sandoval-Insausti, H.; Peñalvo, J.L.; Moreno Iribas, M.C.; Amiano, P.; Bes-Rastrollo, M.; Molina-Montes, E.; Moreno-Franco, B.; Agudo, A.; Mayo, C.L.; et al. Olive oil consumption is associated with a lower risk of cardiovascular disease and stroke. Clin. Nutr. 2022, 41, 122–130. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Flammer, A.J.; Hermann, F.; Sudano, I.; Spieker, L.; Hermann, M.; Cooper, K.A.; Serafini, M.; Lüscher, T.F.; Ruschitzka, F.; Noll, G.; et al. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation 2007, 116, 2376–2382. [Google Scholar] [CrossRef] [Green Version]
- Tanghe, A.; Heyman, E.; Vanden Wyngaert, K.; Van Ginckel, A.; Celie, B.; Rietzschel, E.; Calders, P.; Shadid, S. Evaluation of blood pressure lowering effects of cocoa flavanols in diabetes mellitus: A systematic review and meta-analysis. J. Funct. Foods 2021, 79, 104399. [Google Scholar] [CrossRef]
- Tanghe, A.; Celie, B.; Shadid, S.; Rietzschel, E.; Op ‘t Roodt, J.; Reesink, K.D.; Heyman, E.; Calders, P. Acute Effects of Cocoa Flavanols on Blood Pressure and Peripheral Vascular Reactivity in Type 2 Diabetes Mellitus and Essential Hypertension: A Protocol for an Acute, Randomized, Double-Blinded, Placebo-Controlled Cross-Over Trial. Front. Cardiovasc. Med. 2021, 8, 602086. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2809. [Google Scholar] [CrossRef] [Green Version]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Dinda, B.; Silsarma, I.; Dinda, M.; Rudrapaul, P. Oroxylum indicum (L.) Kurz, an important Asian traditional medicine: From traditional uses to scientific data for its commercial exploitation. J. Ethnopharmacol. 2015, 161, 255–278. [Google Scholar] [CrossRef]
- Lu, Q.-Y.; Zhang, L.; Moro, A.; Chen, M.C.; Harris, D.M.; Eibl, G.; Go, V.-L.W. Detection of Baicalin Metabolites Baicalein and Oroxylin-A in Mouse Pancreas and Pancreatic Xenografts. Tissue Eng. 2007, 23, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Ran, Y.; Qie, S.; Gao, F.; Ding, Z.; Yang, S.; Tian, G.; Liu, Z.; Xi, J. Baicalein ameliorates ischemic brain damage through suppressing proinflammatory microglia polarization via inhibiting the TLR4/NF-κB and STAT1 pathway. Brain Res. 2021, 1770, 147626. [Google Scholar] [CrossRef]
- Song, J.W.; Long, J.Y.; Xie, L.; Zhang, L.L.; Xie, Q.X.; Chen, H.J.; Deng, M.; Li, X.F. Applications, phytochemistry, pharmacological effects, pharmacokinetics, toxicity of Scutellaria baicalensis Georgi. And its probably potential therapeutic effects on COVID-19: A review. Chin. Med. 2020, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Marković, Z.S.; Dimitrić Marković, J.M.; Milenković, D.; Filipović, N. Mechanistic study of the structure-activity relationship for the free radical scavenging activity of baicalein. J. Mol. Model. 2011, 17, 2575–2584. [Google Scholar] [CrossRef] [PubMed]
- Salleh, N.N.H.N.; Othman, F.A.; Kamarudin, N.A.; Tan, S.C. The Biological Activities and Therapeutic Potentials of Baicalein Extracted from Oroxylum indicum: A Systematic Review. Molecules 2020, 25, 5677. [Google Scholar] [CrossRef]
- Zhao, Z.; Nian, M.; Qiao, H.; Yang, X.; Wu, S.; Zheng, X. Review of bioactivity and structure-activity relationship on baicalein (5,6,7-trihydroxyflavone) and wogonin (5,7-dihydroxy-8-methoxyflavone) derivatives: Structural modifications inspired from flavonoids in Scutellaria baicalensis. Eur. J. Med. Chem. 2022, 243, 114733. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef] [Green Version]
- Costine, B.; Zhang, M.; Chhajed, S.; Pearson, B.; Chen, S.; Nadakuduti, S.S. Exploring native Scutellaria species provides insight into differential accumulation of flavones with medicinal properties. Sci. Rep. 2022, 12, 13201. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Bae, J.S. Baicalin, baicalein and wogonin inhibits high glucose-induced vascular inflammation in vitro and in vivo. BMB Rep. 2015, 48, 519–524. [Google Scholar] [CrossRef]
- Baradaran Rahimi, V.; Askari, V.R.; Hosseinzadeh, H. Promising influences of Scutellaria baicalensis and its two active constituents, baicalin, and baicalein, against metabolic syndrome: A review. Phytother. Res. 2021, 35, 3558–3574. [Google Scholar] [CrossRef]
- Zhang, B.W.; Li, X.; Sun, W.L.; Xing, Y.; Xiu, Z.L.; Zhuang, C.L.; Dong, Y.S. Dietary Flavonoids and Acarbose Synergistically Inhibit α-Glucosidase and Lower Postprandial Blood Glucose. J. Agric. Food Chem. 2017, 65, 8319–8330. [Google Scholar] [CrossRef]
- Froldi, G.; Djeujo, F.M.; Bulf, N.; Caparelli, E.; Ragazzi, E. Comparative Evaluation of the Antiglycation and Anti-α-Glucosidase Activities of Baicalein, Baicalin (Baicalein 7-O-Glucuronide) and the Antidiabetic Drug Metformin. Pharmaceutics 2022, 14, 2141. [Google Scholar] [CrossRef]
- Kwak, S.; Ku, S.K.; Han, M.S.; Bae, J.S. Vascular barrier protective effects of baicalin, baicalein and wogonin in vitro and in vivo. Toxicol. Appl. Pharmacol. 2014, 281, 30–38. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Y.; Liu, W.; Liu, Y.; Afzal, S.; Grover, J.; Chang, D.; Münch, G.; Li, C.G.; Lin, S.; et al. Protective effect of the curcumin-baicalein combination against macrovascular changes in diabetic angiopathy. Frontiers in Endocrinology 2022, 13, 953305. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; Hassan, N.A.; Mahmoud, M.F.; Fahmy, A. Baicalein protects against hypertension associated with diabetes: Effect on vascular reactivity and stiffness. Phytomedicine 2014, 21, 1742–1745. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, W.; Yu, N.; Sun, J.; Yu, X.; Li, X.; Xing, Y.; Yan, D.; Ding, Q.; Xiu, Z.; et al. Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats. J. Funct. Foods 2018, 46, 256–267. [Google Scholar] [CrossRef]
- Bitew, M.; Desalegn, T.; Demissie, T.B.; Belayneh, A.; Endale, M.; Eswaramoorthy, R. Pharmacokinetics and drug-likeness of antidiabetic flavonoids: Molecular docking and DFT study. PLoS ONE 2021, 16, e0260853. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Ruan, W.; Wan, X.; lv, C.; He, L.; Lu, L.; Guo, X. Targeting inflammation-associated AMPK//Mfn-2/MAPKs signaling pathways by baicalein exerts anti-atherosclerotic action. Phytother. Res. 2021, 35, 4442–4455. [Google Scholar] [CrossRef]
- Wan, C.X.; Xu, M.; Huang, S.H.; Wu, Q.Q.; Yuan, Y.; Deng, W.; Tang, Q.Z. Baicalein protects against endothelial cell injury by inhibiting the TLR4/NF-κB signaling pathway. Mol. Med. Rep. 2018, 17, 3085–3091. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Miao, Z.; Hu, Y.; Yuan, Y.; Zhou, Y.; Wei, L.; Zhao, K.; Guo, Q.; Lu, N. Baicalein reduces angiogenesis in the inflammatory microenvironment via inhibiting the expression of AP-1. Oncotarget 2017, 8, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Tsai, K.L.; Hung, C.H.; Chan, S.H.; Shih, J.Y.; Cheng, Y.H.; Tsai, Y.J.; Lin, H.C.; Chu, P.M. Baicalein protects against oxLDL-caused oxidative stress and inflammation by modulation of AMPK-alpha. Oncotarget 2016, 7, 72458–72468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machha, A.; Achike, F.I.; Mohd, M.A.; Mustafa, M.R. Baicalein impairs vascular tone in normal rat aortas: Role of superoxide anions. Eur. J. Pharmacol. 2007, 565, 144–150. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Su, Y.L.; Lau, C.W.; Law, W.I.; Huang, Y. Endothelium-dependent contraction and direct relaxation induced by baicalein in rat mesenteric artery. Eur. J. Pharmacol. 1999, 374, 41–47. [Google Scholar] [CrossRef]
- Machha, A.; Mustafa, M.R. Chronic treatment with flavonoids prevents endothelial dysfunction in spontaneously hypertensive rat aorta. J. Cardiovasc. Pharmacol. 2005, 46, 36–40. [Google Scholar] [CrossRef]
- Peng, C.Y.; Pan, S.L.; Huang, Y.W.; Guh, J.H.; Chang, Y.L.; Teng, C.M. Baicalein attenuates intimal hyperplasia after rat carotid balloon injury through arresting cell-cycle progression and inhibiting ERK, Akt, and NF-κB activity in vascular smooth-muscle cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 378, 579–588. [Google Scholar] [CrossRef]
- Sulistyowati, E.; Hsu, J.H.; Lee, S.J.; Huang, S.E.; Sihotang, W.Y.; Wu, B.N.; Dai, Z.K.; Lin, M.C.; Yeh, J.L. Potential Actions of Baicalein for Preventing Vascular Calcification of Smooth Muscle Cells In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 5673. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, H.; Lou, K.; Luo, H.; Hao, S.; Yuan, J.; Liu, Z.; Dong, R. Safety, tolerability, and pharmacokinetics of oral baicalein tablets in healthy Chinese subjects: A single-center, randomized, double-blind, placebo-controlled multiple-ascending-dose study. Clin. Transl. Sci. 2021, 14, 2017–2024. [Google Scholar] [CrossRef]
- Dong, R.; Li, L.; Gao, H.; Lou, K.; Luo, H.; Hao, S.; Yuan, J.; Liu, Z. Safety, tolerability, pharmacokinetics, and food effect of baicalein tablets in healthy Chinese subjects: A single-center, randomized, double-blind, placebo-controlled, single-dose phase I study. J. Ethnopharmacol. 2021, 274, 114052. [Google Scholar] [CrossRef]
- WHO Global Database VigiBase. Available online: https://www.vigiaccess.org/ (accessed on 28 September 2022).
- Pang, H.; Xue, W.; Shi, A.; Li, M.; Li, Y.; Cao, G.; Yan, B.; Dong, F.; Xiao, W.; He, G.; et al. Multiple-Ascending-Dose Pharmacokinetics and Safety Evaluation of Baicalein Chewable Tablets in Healthy Chinese Volunteers. Clin. Drug Investig. 2016, 36, 713–724. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, G.; Zhang, Y.; Wu, X.; Liu, B.; Liu, Y.; Yang, Q.; Du, W.; Liang, J.; Hu, J.; et al. Inhibition of Human UDP-Glucuronosyltransferases1A1–Mediated Bilirubin Glucuronidation by the Popular Flavonoids Baicalein, Baicalin, and Hyperoside Is Responsible for Herb (Shuang-Huang-Lian)-Induced JaundiceS. Drug Metab. Dispos. 2022, 50, 552–565. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [Green Version]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Wongeakin, N.; Bhattarakosol, P.; Patumraj, S. Molecular mechanisms of curcumin on diabetes-induced endothelial dysfunctions: Txnip, ICAM-1, and NOX2 expressions. BioMed. Res. Int. 2014, 2014, 12–14. [Google Scholar] [CrossRef] [Green Version]
- Rungseesantivanon, S.; Thenchaisri, N.; Ruangvejvorachai, P.; Patumraj, S. Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement. Altern. Med. 2010, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Karimian, M.S.; Pirro, M.; Johnston, T.P.; Majeed, M.; Sahebkar, A. Curcumin and Endothelial Function: Evidence and Mechanisms of Protective Effects. Curr. Pharm. Des. 2017, 23, 2462–2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, S.M.; Choi, J.H.; Yoo, D.Y.; Kim, W.; Jung, H.Y.; Kim, J.W.; Yoo, M.; Lee, S.; Kim, C.J.; Yoon, Y.S.; et al. Effects of Curcumin (Curcuma longa) on Learning and Spatial Memory as Well as Cell Proliferation and Neuroblast Differentiation in Adult and Aged Mice by Upregulating Brain-Derived Neurotrophic Factor and CREB Signaling. J. Med. Food 2014, 17, 641–649. [Google Scholar] [CrossRef]
- De Lorenzi, E.; Contardi, C.; Serra, M.; Bisceglia, F.; Franceschini, D.; Pagetta, A.; Zusso, M.; Di Martino, R.M.C.; Seghetti, F.; Belluti, F. Modulation of Amyloid β-induced Microglia Activation and Neuronal Cell Death by Curcumin and Analogues. Int. J. Mol. Sci. 2022, 23, 4381. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zheng, X.; Tang, H.; Zhao, L.; He, C.; Zou, Y.; Song, X.; Li, L.; Yin, Z.; Ye, G. A network pharmacology approach to identify the mechanisms and molecular targets of curcumin against Alzheimer disease. Medicine 2022, 101, e30194. [Google Scholar] [CrossRef] [PubMed]
- Gordon, O.N.; Luis, P.B.; Sintim, H.O.; Schneider, C. Unraveling curcumin degradation: Autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione. J. Biol. Chem. 2015, 290, 4817–4828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Du, Z.; Wang, W.; Song, M.; Sanidad, K.; Sukamtoh, E.; Zheng, J.; Tian, L.; Xiao, H.; Liu, Z.; et al. Structure-Activity Relationship of Curcumin: Role of the Methoxy Group in Anti-inflammatory and Anticolitis Effects of Curcumin. J. Agric. Food Chem. 2017, 65, 4509–4515. [Google Scholar] [CrossRef]
- Avci, G.; Kadioglu, H.; Sehirli, A.O.; Bozkurt, S.; Guclu, O.; Arslan, E.; Muratli, S.K. Curcumin protects against ischemia/reperfusion injury in rat skeletal muscle. J. Surg. Res. 2012, 172, e39–e46. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Shen, Y.; Tan, T.; Xie, N.; Luo, M.; Li, Z.; Xie, X. Curcumin ameliorates ischemia-induced limb injury through immunomodulation. Med. Sci. Monit. 2016, 22, 2035–2042. [Google Scholar] [CrossRef] [Green Version]
- You, J.; Sun, J.; Ma, T.; Yang, Z.; Wang, X.; Zhang, Z.; Li, J.; Wang, L.; Ii, M.; Yang, J.; et al. Curcumin induces therapeutic angiogenesis in a diabetic mouse hindlimb ischemia model via modulating the function of endothelial progenitor cells. Stem Cell Res. Ther. 2017, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, Q.; Rao, G.; Qiu, J.; He, R. Curcumin improves perfusion recovery in experimental peripheral arterial disease by upregulating microRNA-93 expression. Exp. Ther. Med. 2019, 17, 798–802. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, S.; Yao, Y.H.; Zhang, Z.M.; Liu, J.S.; Xiang, H. Curcumin inhibits HIF-1α induced functional changes of macrophages. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1816–1825. [Google Scholar]
- Alizadeh, M.; Kheirouri, S. Curcumin against advanced glycation end products (AGEs) and AGEs-induced detrimental agents. Crit. Rev. Food Sci. Nutr. 2019, 59, 1169–1177. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, A. Laboratory Investigation. Lab. Investig. 2014, 94, 503–516. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, A.; Atkin, S.L.; Nikiforov, N.G.; Sahebkar, A. Therapeutic Role of Curcumin in Diabetes: An Analysis Based on Bioinformatic Findings. Nutrients 2022, 14, 3244. [Google Scholar] [CrossRef]
- Changal, K.H.; Khan, M.S.; Bashir, R.; Sheikh, M.A. Curcumin Preparations Can Improve Flow-Mediated Dilation and Endothelial Function: A Meta-Analysis. Complement. Med. Res. 2020, 27, 272–281. [Google Scholar] [CrossRef]
- Alidadi, M.; Liberale, L.; Montecucco, F.; Majeed, M.; Al-Rasadi, K.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. Protective Effects of Curcumin on Endothelium: An Updated Review. Adv. Exp. Med. Biol. 2021, 1291, 103–119. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [Green Version]
- Witika, B.A.; Makoni, P.A.; Matafwali, S.K.; Mweetwa, L.L.; Shandele, G.C.; Walker, R.B. Enhancement of biological and pharmacological properties of an encapsulated polyphenol: Curcumin. Molecules 2021, 26, 4244. [Google Scholar] [CrossRef]
- Poole, K.M.; Nelson, C.E.; Joshi, R.V.; Martin, J.R.; Gupta, M.K.; Haws, S.C.; Kavanaugh, T.E.; Skala, M.C.; Duvall, C.L. ROS-responsive microspheres for on demand antioxidant therapy in a model of diabetic peripheral arterial disease. Biomaterials 2015, 41, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.; Noh, J.; Kang, C.; Yoo, D.; Song, C.; Lee, D. Ultrasound imaging and on-demand therapy of peripheral arterial diseases using H2O2-Activated bubble generating anti-inflammatory polymer particles. Biomaterials 2018, 179, 175–185. [Google Scholar] [CrossRef]
- Verma, K.; Tarafdar, A.; Kumar, D.; Kumar, Y.; Rana, J.S.; Badgujar, P.C. Formulation and characterization of nano-curcumin fortified milk cream powder through microfluidization and spray drying. Food Res. Int. 2022, 160, 111705. [Google Scholar] [CrossRef]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef]
- Goel, A.; Jhurani, S.; Aggarwal, B.B. Multi-targeted therapy by curcumin: How spicy is it? Mol. Nutr. Food Res. 2008, 52, 1010–1030. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phytother. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Surma, S.; Sahebkar, A.; Urbański, J.; Penson, P.E.; Banach, M. Curcumin-The Nutraceutical with Pleiotropic Effects? Which Cardiometabolic Subjects Might Benefit the Most? Front. Nutr. 2022, 9, 865497. [Google Scholar] [CrossRef]
- Lombardi, N.; Crescioli, G.; Maggini, V.; Ippoliti, I.; Menniti-Ippolito, F.; Gallo, E.; Brilli, V.; Lanzi, C.; Mannaioni, G.; Firenzuoli, F.; et al. Acute liver injury following turmeric use in Tuscany: An analysis of the Italian Phytovigilance database and systematic review of case reports. Br. J. Clin. Pharmacol. 2021, 87, 741–753. [Google Scholar] [CrossRef]
- Haynes, L.J.; Taylor, D.R. C-glycosyl compounds. Part V. Mangiferin; the nuclear magnetic resonance spectra of xanthones. J. Chem. Soc. C Org. 1966, 1685–1687. [Google Scholar] [CrossRef]
- Saha, S.; Sadhukhan, P.; Sil, P.C. Mangiferin: A xanthonoid with multipotent anti-inflammatory potential. BioFactors 2016, 42, 459–474. [Google Scholar] [CrossRef]
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; De Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017, 16, 84. [Google Scholar] [CrossRef]
- Benard, O.; Chi, Y. Medicinal Properties of Mangiferin, Structural Features, Derivative Synthesis, Pharmacokinetics and Biological Activities. Mini-Rev. Med. Chem. 2015, 15, 582–594. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, J.; Roy, A.; Sil, P.C. Mangiferin exerts hepatoprotective activity against D-galactosamine induced acute toxicity and oxidative/nitrosative stress via Nrf2-NFκB pathways. Toxicol. Appl. Pharmacol. 2012, 260, 35–47. [Google Scholar] [CrossRef]
- Pal, P.B.; Sinha, K.; Sil, P.C. Mangiferin, a Natural Xanthone, Protects Murine Liver in Pb(II) Induced Hepatic Damage and Cell Death via MAP Kinase, NF-κB and Mitochondria Dependent Pathways. PLoS ONE 2013, 8, e56894. [Google Scholar] [CrossRef] [Green Version]
- Andreu, G.P.; Delgado, R.; Velho, J.A.; Curti, C.; Vercesi, A.E. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe 2+-citrate. Eur. J. Pharmacol. 2005, 513, 47–55. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Hou, F.; Wang, X.; Liu, B. Mangiferin inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/NLRP3 inflammasome activation with regulation of AMPK in endothelial cells. Metab. Clin. Exp. 2015, 64, 428–437. [Google Scholar] [CrossRef]
- He, Q.; Ai, J.; Huang, Y. [Relationship between endothelial damage and p120-catenin in paraquat intoxication and the protective effect of mangiferin]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2014, 26, 369–373. [Google Scholar] [CrossRef]
- Venugopal, R.; Sakthisekaran, D.; Rajkapoor, B.; Nishigaki, I. In vitro protective effect of mangiferin against glycated protein-iron chelate induced toxicity in human umbelical vein endothelial cells. J. Biol. Sci. 2007, 7, 1227–1232. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Lv, H.; Tang, C.; Li, Y.; Huang, J.; Zhang, H. Mangiferin inhibits cell migration and angiogenesis via PI3K/AKT/mTOR signaling in high glucose- and hypoxia-induced RRCECs. Mol. Med. Rep. 2021, 23, 473. [Google Scholar] [CrossRef]
- Daud, N.H.; Aung, C.S.; Hewavitharana, A.K.; Wilkinson, A.S.; Pierson, J.T.; Roberts-Thomson, S.J.; Shaw, P.N.; Monteith, G.R.; Gidley, M.J.; Parat, M.O. Mango extracts and the mango component mangiferin promote endothelial cell migration. J. Agric. Food Chem. 2010, 58, 5181–5186. [Google Scholar] [CrossRef]
- Hou, J.; Zheng, D.; Fung, G.; Deng, H.; Chen, L.; Liang, J.; Jiang, Y.; Hu, Y. Mangiferin suppressed advanced glycation end products (AGEs) through NF-κB deactivation and displayed anti-inflammatory effect in streptozotocin and high fat diet-diabetic cardiomyopathy rats. Can. J. Physiol. Pharmacol. 2016, 94, 332–340. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, D.L.; Jia, M.; Hao, W.H.; Li, Y. jun Mangiferin inhibits high-fat diet induced vascular injury via regulation of PTEN/AKT/eNOS pathway. J. Pharmacol. Sci. 2018, 137, 265–273. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, W.; Yang, T.; Li, M.; Qin, L.; Wu, L.; Liu, T. Oral administration of mangiferin ameliorates diabetes in animal models: A meta-analysis and systematic review. Nutr. Res. 2021, 87, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Dorighello, G.G.; Inada, N.M.; Paim, B.A.; Pardo-Andreu, G.L.; Vercesi, A.E.; Oliveira, H.C.F. Mangifera indica L. extract (Vimang®) reduces plasma and liver cholesterol and leucocyte oxidative stress in hypercholesterolemic LDL receptor deficient mice. Cell Biol. Int. 2018, 42, 747–753. [Google Scholar] [CrossRef] [PubMed]
- RamPravinKumar, M.; Dhananjayan, K. Peripheral arterial disease: Effects of ethanolic extracts of seed kernels of mango (Mangifera indica L.) on acute hind limb ischemia-reperfusion injury in diabetic rats. J. Tradit. Complement. Med. 2021, 11, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Na, L.; Zhang, Q.; Jiang, S.; Du, S.; Zhang, W.; Li, Y.; Sun, C.; Niu, Y. Mangiferin supplementation improves serum lipid profiles in overweight patients with hyperlipidemia: A double-blind randomized controlled trial. Sci. Rep. 2015, 5, 10344. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Wang, F.; Li, Y.; Li, Y.; Wang, M.; Sun, D.; Sun, C. Pharmacokinetic study of mangiferin in human plasma after oral administration. Food Chem. 2012, 132, 289–294. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Magar, R.T.; Sohng, J.K. A Review on Structure, Modifications and Structure-Activity Relation of Quercetin and Its Derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. O-Glycoside quercetin derivatives: Biological activities, mechanisms of action, and structure–activity relationship for drug design, a review. Phytother. Res. 2022, 36, 778–807. [Google Scholar] [CrossRef]
- Babenkova, I.V.; Osipov, A.N.; Teselkin, Y.O. The Effect of Dihydroquercetin on Catalytic Activity of Iron (II) Ions in the Fenton Reaction. Bull. Exp. Biol. Med. 2018, 165, 347–350. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytotherapy Research 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Pérez-Vizcaíno, F.; Zarzuelo, A.; Jiménez, J.; Tamargo, J. Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur. J. Pharmacol. 1993, 239, 1–7. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Oue, E. Antihypertensive effect of quercetin in rats fed with a high-fat high-sucrose diet. Biosci. Biotechnol. Biochem. 2006, 70, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.K.; Pace-Asciak, C.R. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen. Pharmacol. Vasc. Syst. 1996, 27, 363–366. [Google Scholar] [CrossRef]
- Duarte, J.; Pérez-Palencia, R.; Vargas, F.; Angeles Ocete, M.; Pérez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.X.; Xing, N.Z.; Cao, X. guang Quercetin inhibits choroidal and retinal angiogenesis in vitro. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 373–378. [Google Scholar] [CrossRef]
- Igura, K.; Ohta, T.; Kuroda, Y.; Kaji, K. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett. 2001, 171, 11–16. [Google Scholar] [CrossRef]
- Tan, W.F.; Lin, L.P.; Li, M.H.; Zhang, Y.X.; Tong, Y.G.; Xiao, D.; Ding, J. Quercetin, a dietary-derived flavonoid, possesses antiangiogenic potential. Eur. J. Pharmacol. 2003, 459, 255–262. [Google Scholar] [CrossRef]
- Suganya, N.; Bhakkiyalakshmi, E.; Suriyanarayanan, S.; Paulmurugan, R.; Ramkumar, K.M. Quercetin ameliorates tunicamycin-induced endoplasmic reticulum stress in endothelial cells. Cell Prolif. 2014, 47, 231–240. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, D.; Wang, J.; Pang, X.; Liu, H. Protective Effect of Quercetin against Oxidative Stress-Induced Cytotoxicity in Rat Pheochromocytoma (PC-12) Cells. Molecules 2017, 22, 1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, P.A.; Braune, A.; Hölzlwimmer, G.; Quintanilla-Fend, L.; Haller, D. Quercetin inhibits TNF-induced NF-κB transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells. J. Nutr. 2007, 137, 1208–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.K.; Gao, J.; Zhu, D.N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef] [Green Version]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Popiolek-Kalisz, J.; Fornal, E. The effects of quercetin supplementation on blood pressure–meta-analysis. Curr. Probl. Cardiol. 2022, 47, 101350. [Google Scholar] [CrossRef]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Liao, D.; Dong, Y.; Pu, R. Effect of quercetin supplementation on plasma lipid profiles, blood pressure, and glucose levels: A systematic review and meta-analysis. Nutr. Rev. 2020, 78, 615–626. [Google Scholar] [CrossRef]
- Bradamante, S.; Barenghi, L.; Villa, A. Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev. 2004, 22, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Ragazzi, E.; Froldi, G.; Fassina, G. Resveratrol activity on guinea pig isolated trachea from normal and albumin-sensitized animals. Pharmacol. Res. Commun. 1988, 20, 79–82. [Google Scholar] [CrossRef]
- Pervaiz, S. Resveratrol: From grapevines to mammalian biology. FASEB J. 2003, 17, 1975–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppa, T.; Lazzè, M.C.; Cazzalini, O.; Perucca, P.; Pizzala, R.; Bianchi, L.; Stivala, L.A.; Forti, L.; MacCario, C.; Vannini, V.; et al. Structure-Activity relationship of resveratrol and its analogue, 4,4′-dihydroxy-trans-stilbene, toward the endothelin axis in human endothelial cells. J. Med. Food 2011, 14, 1173–1180. [Google Scholar] [CrossRef]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific Structural Determinants Are Responsible for the Antioxidant Activity and the Cell Cycle Effects of Resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef] [Green Version]
- Plauth, A.; Geikowski, A.; Cichon, S.; Wowro, S.J.; Liedgens, L.; Rousseau, M.; Weidner, C.; Fuhr, L.; Kliem, M.; Jenkins, G.; et al. Hormetic shifting of redox environment by pro-oxidative resveratrol protects cells against stress. Free. Radic. Biol. Med. 2016, 99, 608–622. [Google Scholar] [CrossRef] [Green Version]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [Green Version]
- Buluc, M.; Demirel-Yilmaz, E. Resveratrol decreases calcium sensitivity of vascular smooth muscle and enhances cytosolic calcium increase in endothelium. Vasc. Pharmacol. 2006, 44, 231–237. [Google Scholar] [CrossRef]
- Jäger, U.; Nguyen-Duong, H. Relaxant effect of trans-resveratrol on isolated porcine coronary arteries. Arzneimittelforschung 1999, 49, 207–211. [Google Scholar] [CrossRef]
- El-Mowafy, A.M. Resveratrol activates membrane-bound guanylyl cyclase in coronary arterial smooth muscle: A novel signaling mechanism in support of coronary protection. Biochem. Biophys. Res. Commun. 2002, 291, 1218–1224. [Google Scholar] [CrossRef]
- Naderali, E.K.; Doyle, P.J.; Williams, G. Resveratrol induces vasorelaxation of mesenteric and uterine arteries from female guinea-pigs. Clin. Sci. (Lond. Engl. 1979) 2000, 98, 537–543. [Google Scholar] [CrossRef]
- Klinge, C.M.; Wickramasinghe, N.S.; Ivanova, M.M.; Dougherty, S.M. Resveratrol stimulates nitric oxide production by increasing estrogen receptor αa-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008, 22, 2185–2197. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Zhang, R.; Ye, X.; Zhang, C.; Jin, X.; Yamori, Y.; Hao, L.; Sun, X.; Ying, C. Resveratrol ameliorates high-glucose-induced hyperpermeability mediated by caveolae via VEGF/KDR pathway. Genes Nutr. 2013, 8, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jiang, C.; Zhang, J.; Liu, B.; Du, Q. Resveratrol inhibits inflammation and ameliorates insulin resistant endothelial dysfunction via regulation of AMP-activated protein kinase and sirtuin 1 activities. J. Diabetes 2016, 8, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Smith, K.; Labinskyy, N.; Orosz, Z.; Rivera, A.; Ungvari, Z. Resveratrol attenuates TNF-α-induced activation of coronary arterial endothelial cells: Role of NF-κB inhibition. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, C.E.; Kim, M.H. Flavonoids inhibit high glucose-induced up-regulation of ICAM-1 via the p38 MAPK pathway in human vein endothelial cells. Biochem. Biophys. Res. Commun. 2011, 415, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhu, S.; Chang, S.; Cao, Y.; Dong, J.; Li, J.; Long, R.; Zhou, Y. Protective effects of chronic resveratrol treatment on vascular inflammatory injury in streptozotocin-induced type 2 diabetic rats: Role of NF-kappa B signaling. Eur. J. Pharmacol. 2013, 720, 147–157. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiology. Heart Circ. Physiol. 2010, 299, H18-24. [Google Scholar] [CrossRef] [Green Version]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Et Biophys. Acta Mol. Basis Dis. 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef]
- Mohammad, G.; Abdelaziz, G.M.; Siddiquei, M.M.; Ahmad, A.; De Hertogh, G.; Abu El-Asrar, A.M. Cross-Talk between Sirtuin 1 and the Proinflammatory Mediator High-Mobility Group Box-1 in the Regulation of Blood-Retinal Barrier Breakdown in Diabetic Retinopathy. Curr. Eye Res. 2019, 44, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Li, K.X.; Ji, M.J.; Sun, H.J. An updated pharmacological insight of resveratrol in the treatment of diabetic nephropathy. Gene 2021, 780, 145532. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Chi, T.C.; Chuang, L.M.; Su, M.J. Resveratrol enhances insulin secretion by blocking KATP and KV channels of beta cells. Eur. J. Pharmacol. 2007, 568, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kopp, P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the ‘French paradox’? Eur. J. Endocrinol. 1998, 138, 619–620. [Google Scholar] [CrossRef] [Green Version]
- Imamura, H.; Yamaguchi, T.; Nagayama, D.; Saiki, A.; Shirai, K.; Tatsuno, I. Resveratrol ameliorates arterial stiffness assessed by cardio-ankle vascular index in patients with type 2 diabetes mellitus. Int. Heart J. 2017, 58, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Carrizzo, A.; Puca, A.; Damato, A.; Marino, M.; Franco, E.; Pompeo, F.; Traficante, A.; Civitillo, F.; Santini, L.; Trimarco, V.; et al. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension 2013, 62, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. American J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.H.X.; Raederstorff, D.; Howe, P.R.C. Acute resveratrol consumption improves neurovascular coupling capacity in adults with type 2 diabetes mellitus. Nutrients 2016, 8, 425. [Google Scholar] [CrossRef] [Green Version]
- Jeyaraman, M.M.; Al-Yousif, N.S.H.; Singh Mann, A.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 2020, CD011919. [Google Scholar] [CrossRef]
- McDermott, M.M.; Leeuwenburgh, C.; Guralnik, J.M.; Tian, L.; Sufit, R.; Zhao, L.; Criqui, M.H.; Kibbe, M.R.; Stein, J.H.; Lloyd-Jones, D.; et al. Effect of Resveratrol on Walking Performance in Older People with Peripheral Artery Disease: The RESTORE Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 902–907. [Google Scholar] [CrossRef]
- Hyrsova, L.; Vanduchova, A.; Dusek, J.; Smutny, T.; Carazo, A.; Maresova, V.; Trejtnar, F.; Barta, P.; Anzenbacher, P.; Dvorak, Z.; et al. Trans-resveratrol, but not other natural stilbenes occurring in food, carries the risk of drug-food interaction via inhibition of cytochrome P450 enzymes or interaction with xenosensor receptors. Toxicol. Lett. 2019, 300, 81–91. [Google Scholar] [CrossRef]
- Giuliani, C.; Iezzi, M.; Ciolli, L.; Hysi, A.; Bucci, I.; Di Santo, S.; Rossi, C.; Zucchelli, M.; Napolitano, G. Resveratrol has anti-thyroid effects both in vitro and in vivo. Food Chem. Toxicol. 2017, 107, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.D.; Morrissey, R.L.; Usborne, A.L.; Kapetanovic, I.; Crowell, J.A.; Muzzio, M.; McCormick, D.L. Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food Chem. Toxicol. 2011, 49, 3319–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boocock, D.J.; Faust, G.E.S.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [Green Version]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Safety of synthetic trans-resveratrol as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2016, 14, 4368. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Froldi, G.; Ragazzi, E. Selected Plant-Derived Polyphenols as Potential Therapeutic Agents for Peripheral Artery Disease: Molecular Mechanisms, Efficacy and Safety. Molecules 2022, 27, 7110. https://doi.org/10.3390/molecules27207110
Froldi G, Ragazzi E. Selected Plant-Derived Polyphenols as Potential Therapeutic Agents for Peripheral Artery Disease: Molecular Mechanisms, Efficacy and Safety. Molecules. 2022; 27(20):7110. https://doi.org/10.3390/molecules27207110
Chicago/Turabian StyleFroldi, Guglielmina, and Eugenio Ragazzi. 2022. "Selected Plant-Derived Polyphenols as Potential Therapeutic Agents for Peripheral Artery Disease: Molecular Mechanisms, Efficacy and Safety" Molecules 27, no. 20: 7110. https://doi.org/10.3390/molecules27207110