- 1Department of Neurology, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
- 2Department of Neurology, National Institute of Neurology and Neurovascular Diseases, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
- 3MedInst Romanian-German Diagnostic Center, Bucharest, Romania
Cerebrospinal fluid (CSF) leakage is considered the cause of spontaneous intracranial hypotension (SIH), an important etiology for new daily persistent headaches and a potentially life-threatening condition. Minor traumatic events rarely lead to CSF leakage, contrasting with iatrogenic interventions such as a lumbar puncture or spinal surgery, which are commonly complicated by dural tears. Most meningeal lesions are found in the cervicothoracic region, followed by the thoracic region, and rarely in the lumbar region, and extremely rarely in the sacral region. We describe two patients admitted to our hospital for severe headaches aggravated in the orthostatic position, with a recent history of minor trauma and sustained physical effort, respectively. In the first case, a bone fragment pierced an incidental congenital meningocele creating a dural fistula. An extensive extradural CSF collection, spanning the cervicothoracic region (C4–T10), was described in the second case. In both patients, the clinical evolution was favorable under conservative treatment.
Introduction
Tears of the spinal dural membrane, rupture of a meningeal diverticulum, and development of CSF-venous fistulas are the major sources of cerebrospinal fluid leaks (1). Dural tears may occur spontaneously or after deliberate or accidental disruption of the meninges (2, 3). Meningeal diverticula, also known as meningoceles, which may be found at single or at multiple levels simultaneously (4, 5), are frequently associated with connective tissue abnormalities, and develop spontaneously or after the healing of a dural tear (6). CSF leakage is a known cause of spontaneous intracranial hypotension (SIH), an under-diagnosed although not so rare syndrome characterized by a classical triad of low cerebrospinal fluid pressure, orthostatic headache, and brain “sag” with diffuse pachymeningeal enhancement on magnetic resonance imaging (MRI) (7, 8). We present two cases of intracranial hypotension with onset after a minor fall and, sustained carrying effort, respectively.
Case presentation
Patient 1
A 37-year-old previously healthy female patient was admitted to our hospital for severe orthostatic headaches with onset 5 days prior, after a seemingly innocuous fall on the buttocks during a ballet lesson, which she was practicing as a hobby. No history of direct head trauma or whiplash was reported. She reported no back pain. The headache was holocephalic, exacerbated by the orthostatic position, slightly relieved by clinostatism, and it was not associated with neck stiffness, fever, nausea, or vomiting. The physical exam was normal, without sensory or motor deficits, and without incontinence. A CT scan of the brain performed at admission was unremarkable.
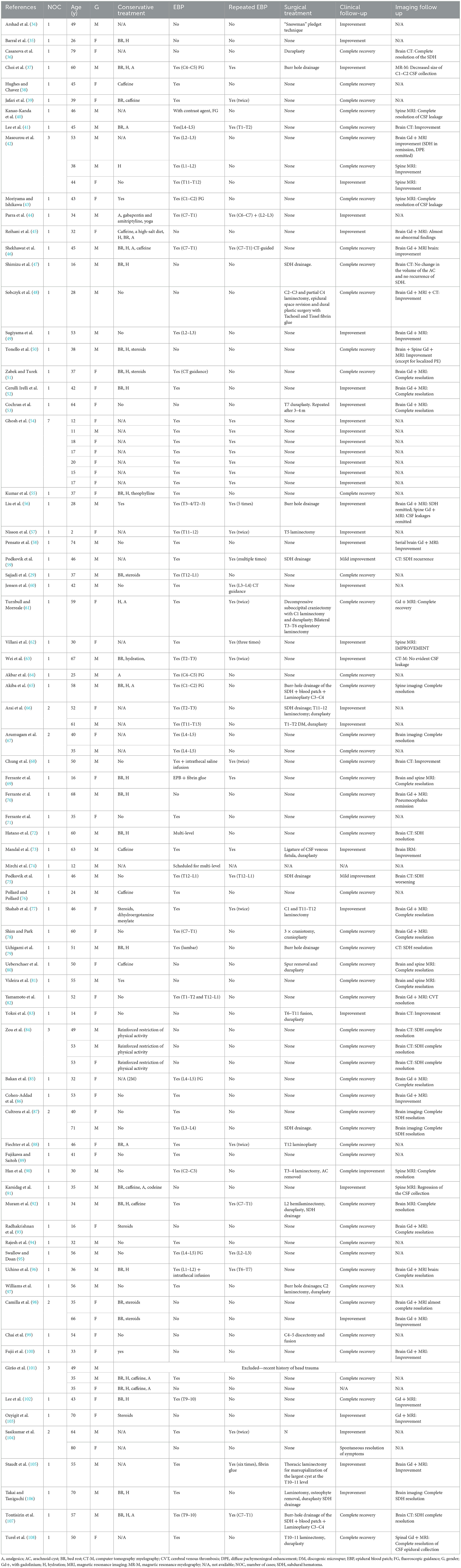
Considering the sensitivity to changes in position, a characteristic element of SIH, a contrast-enhanced MRI of the brain was also performed (Figures 1A–C). The MRI revealed diffuse, relatively uniform thickening and enhancement of pachymeninges, without pathological leptomeningeal enhancement (Figures 1A, B), an enlarged pituitary gland with a cranially convex superior border (Figure 1C) and slightly engorged dural venous sinuses and cortical veins, all findings suggestive of intracranial hypotension syndrome. Otherwise, the brain examination was normal.
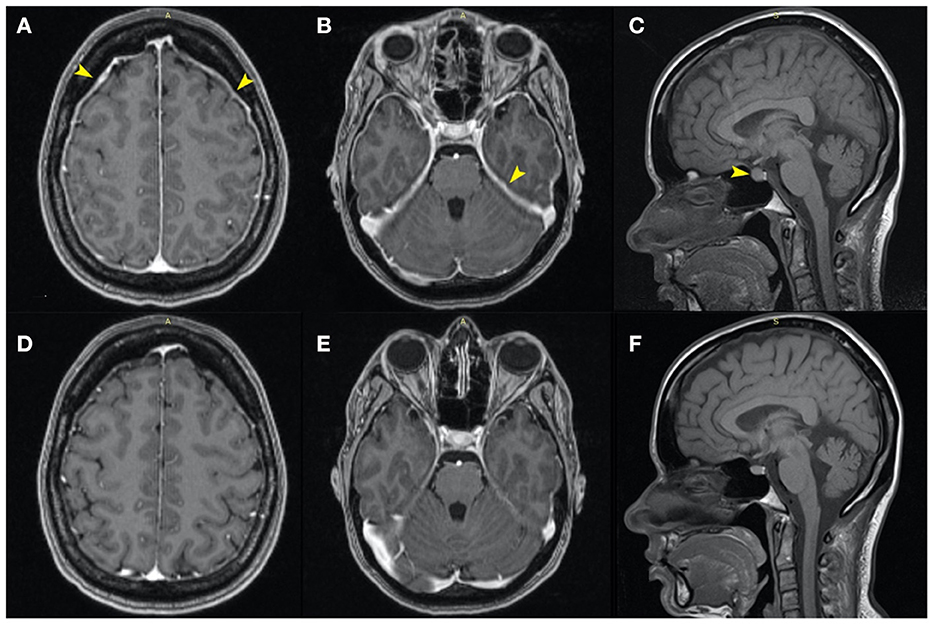
Figure 1. Axial MRI contrast-enhanced T1 images: similar sections at the moment of diagnosis (A–C), and, respectively, 5 months later (D–F). Initially, the MRI revealed diffuse, relatively uniform thickening and enhancement of the pachymeninges and tentorium, without pathological enhancement [(A, B)—yellow arrows] and an enlarged pituitary gland with a cranially convex superior border [(C)—yellow arrow]. Also, the dural venous sinuses and cortical veins were initially slightly engorged. Five months later the images showed normalization of the pachymeninges (D, E) and a reduction of the craniocaudal diameter of the hypophysis (F).
We followed up with a contrast-enhanced MRI of the whole spine to search for a possible dural defect. The cervicothoracic spinal cord morphology and signal were normal. However, the examination revealed anterior and posterior epidural “banded” enhancement of the whole spine more evident at C2–C3 (with a maximum thickness of 6 mm) in the continuity of the infratentorial pachymeningeal thickening, probably reflecting engorgement of epidural venous plexuses. The medullary cone was in a low position (L3), and the terminal filum was thickened and attached posteriorly at L4–L5. There was a wide posterior S3–S4 dysraphism, and bilateral S3 bulky meningocele (32/22/22.5 mm on the right side and 27/16/21 mm on the left side) occupied the sacral canal (Figures 2D, E) exerting significant mass effect with pressure atrophy of the S3, S4 bodies and of posterior vertebral elements. Probably incidentally, two periradicular arachnoid cysts were present on the left S2 and S4 roots (Figure 4). The anterior sacral wall seemed fractured at the S4 level, with a small left anterolateral bone fragment that appeared to protrude internally (Figure 2F). Dural discontinuity of the anteroinferior wall of the left meningocele toward the presacral space was observed as the above-mentioned bone fragment seemed to pierce it. Considering the malformed osseous aspects, the suspected sacral fracture and limited MRI resolution in regard to bone, a computed tomography of the spine was also performed, confirming the S4 fracture extending between both anterior holes with a displaced bony fragment (5/2 mm) protruding into the sacral canal (Figures 2A–C).
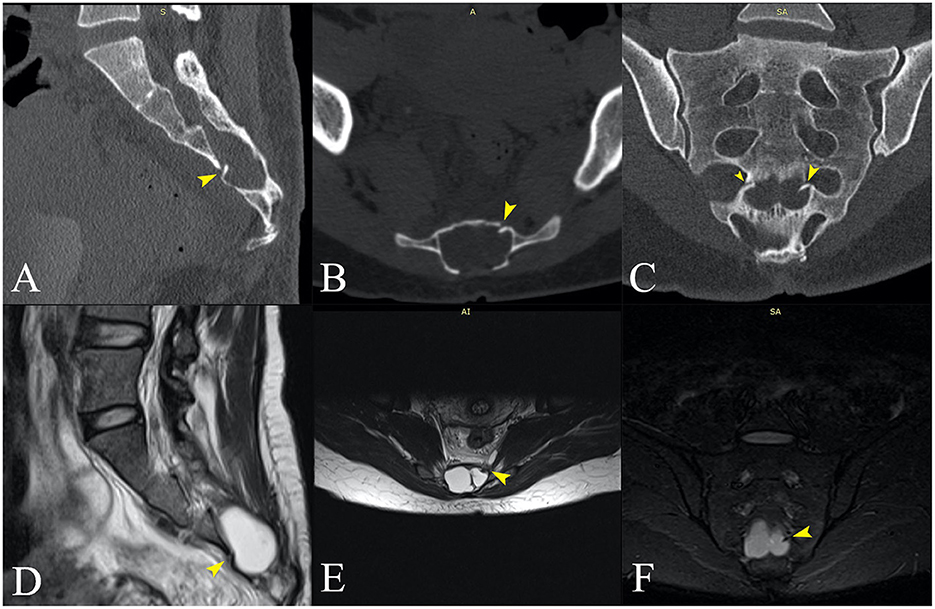
Figure 2. (A–C) Sagittal, axial, and coronal computed tomography, and (D–F) MRI images at approximately the same anatomical plane of section. (D–F) Voluminous meningoceles visible on the MRI images. (B) The posterior dysraphism is clearly visible on the CT transversal section. (C) The fracture line is best visualized in the coronal plane (small and large yellow arrows), with a bone fragment protruding into the sacral canal on the left side [(A–F)—large yellow arrows]. (D, E) The bone fragment clearly imprints the left sacral meningocele and appears to pierce it (F).
After 7 days of symptomatic analgesic treatment, intravenous fluid therapy, and prolonged supine position, the headaches improved significantly. At the 5 months follow-up, the patient had no headache and no neurologic deficits. The brain MRI also showed marked improvement, with the disappearance of the thickening of the pachymeninges (Figures 1D, E) and with a slightly reduced size of the pituitary gland (Figure 1F).
Patient 2
An overweight 38-year-old man, a smoker, presented with frontal and temporal headaches with sudden onset the previous day. The headaches were associated with nausea and vomiting and were relieved by the supine position. After several hours, severe cervical pain was added to the symptomatology. The patient had not suffered any obvious trauma and had no medical history. Still, a few days before the onset of the symptoms, he had made an intense physical effort on a mountain hike when he carried his daughter, weighing about 12 kg, on his shoulders for several hours a day for 2 days in a row. No neck stiffness, fever, or neurological deficits were found during the clinical examination. A CT scan and a DWI-MRI sequence of the brain were performed at the admission, and both were normal. Given the sudden onset and the associated emetic syndrome, a lumbar puncture was performed to rule out a possible subarachnoid hemorrhage. The opening pressure was within normal range, the CSF was clear, and the laboratory results were unremarkable.
Next, we ordered a contrast-enhanced MRI of the brain, which revealed mild diffuse cortical vein engorgement visible on SWI, minimal diffuse thickening of the cerebellar tentorium and pericerebral pachymeninges (especially bilateral paramedian parietal) (Figure 3A), and an enlarged pituitary gland with superior border cranially convex (Figure 3B), all of which suggested intracranial hypotension. Finally, the patient underwent a spine MRI that showed a posterior cervicothoracic epidural collection with fluid signals similar to the CSF (Figures 3C, D), extending craniocaudally from the right side of C4 to T10, with a maximum thickness of about 5 mm in the thoracic region. The thoracic spinal cord was anteriorly displaced but not compressed. Also, we observed engorgement of the posterior epidural venous plexuses, supporting the diagnosis of intracranial hypotension syndrome. Under conservative treatment with non-steroidal anti-inflammatory and antiemetic drugs, the symptomatology gradually improved. The patient was discharged after 10 days without any complaints or neurological deficits. He did not attend his scheduled follow-up.
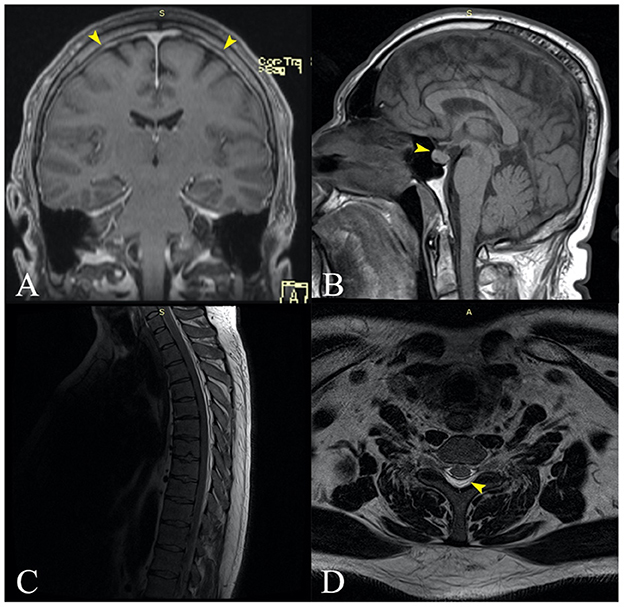
Figure 3. (A) Coronal MRI contrast-enhanced T1 image revealing slight diffuse thickening of the cerebellar tentorium and pericerebral pachymeninges, especially bilateral paramedian parietal [(A)—yellow arrows]. (B) Sagittal MRI contrast-enhanced T1 showing an enlarged pituitary gland with superior border cranially convex [(B)—yellow arrow]. (C) Sagittal spine MRI showing a posterior cervicothoracic epidural collection with fluid signal, extended craniocaudal from the C4 to T10, with a maximum thickness of about 5 mm in the thoracic region. The thoracic spinal cord is anteriorly displaced but not compressed. (D) Transversal MRI section of the thoracic spine (T6) showing the posterior epidural collection that displaces the spinal content anteriorly [(D)—yellow arrow].
Discussion
Low CSF pressure syndromes are classified as primary (or spontaneous) and secondary (9). A CSF leak is described as spontaneous when there is no proceeding history of a major traumatic event or spinal medical procedures (7, 8). Spontaneous CSF leaks have a multifactorial etiology, with three main production mechanisms: meningeal diverticula leakage, spinal ventral dural tears, and cerebrospinal fluid–venous fistula (10). The meningeal diverticula develop along a nerve root sleeve, especially in the thoracic or upper lumbar spine, and may involve large meningeal tears with high-flow CSF leaks or slow-flow leaks facilitated by Valsalva-like maneuvers. Ventral dural tears are most commonly caused by osteophytes or calcified intervertebral disks that incise the dura, producing a longitudinal tear. They are associated with high-flow CSF passage to the epidural spaces, and the protrusion of the spur into the tear may prevent spontaneous healing, requiring surgical treatment. In the cerebrospinal fluid–venous fistula, there is a direct connection between the spinal subarachnoid space and a segmental spinal vein, with rapid loss of CSF following the pressure gradient, which is higher in CSF than in the venous segment. Dynamic CT myelography can detect the pathological filling of the vertebral venous network from the CSF-injected contrast media. Spontaneous improvement is possible in low-flow spinal CSF leakage, while in high-flow and cases with protruding osseous spurs, interventional treatment are often needed (epidural targeted blood patch or neurosurgical closure of the defect) (11).
In about one-third of the patients, a history of mild trauma, such as coughing, sneezing, or lifting can be noted (5, 8). Two-thirds of the patients diagnosed with spontaneous CSF leaks and meningeal diverticula suffer from a generalized connective tissue disorder (5).
Secondary CSF leaks are caused by invasive procedures, such as spinal tap, myelography, spinal surgery, pneumonectomy, or by major traumatic events (9). They can also be associated with other underlying medical conditions, such as degenerative pathology of the spine, arteriosclerosis, or dehydration (2, 10).
The principal consequence of CSF leaks is SIH. This syndrome has an incidence of 2–5/100,000/year with a peak age of 30–50 years and female predominance. It is characterized by the classic triad of low CSF pressure, orthostatic headache, and brain “sag” with a diffuse pachymeningeal MRI enhancement (7, 12). In our first case, the patient suffered a minor accident by falling during a ballet lesson. The patient had an undiagnosed malformation of the sacral spine (wide posterior S3–S4 dysraphism and bilateral S3 meningoceles) which, combined with the minor trauma, proved causal. The fall produced a transverse S4 fracture, from which an osseous fragment seems to have pierced the dura (Figure 4). Sacral fractures are uncommon injuries that most often result from high-energy trauma such as motor vehicle accidents and falls from an elevation (14). Only 5% of sacral fractures occur in isolation, 45% occur with a concomitant pelvic ring injury, and up to 50% are associated with neurological injuries (14, 15). Low transverse fractures, at the level of S3 or below, typically result after a fall onto the buttocks, as was the case in our patient. Isolated fractures in the lumbosacral region may rarely be complicated with dural injury, causing CSF leakage and formation of a traumatic pseudomeningocele (16–18).
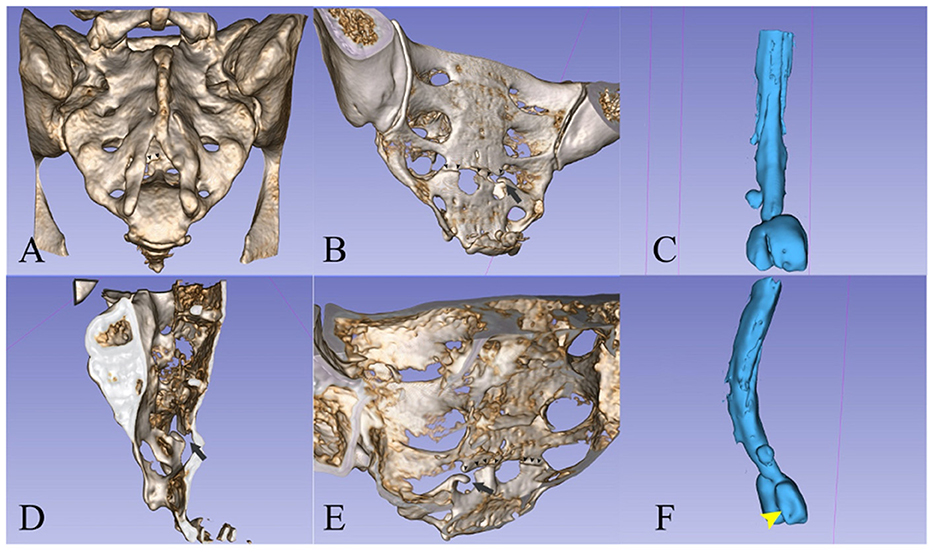
Figure 4. (A, B, D, E) 3D reconstruction (13) of the sacrum from the CT scan images. (C, F) A spinal canal mold from the CSF signal on the MRI images. The left S2 radicular cyst is visible above the meningoceles. (A) Posterior view showing the dysraphism at the level of the bone. [(A)—small arrowheads] The fracture line is partially visible. [(B)—small arrowheads] Posterior oblique view of the pelvic surface of the sacrum showing the transverse fracture at S4 and [(B)—black arrow] the bone fragment that protrudes into the spinal canal. (C) The spinal canal ends with two large meningoceles at the S3 level. (D) Interior view of the sacral canal from the midline, the black arrow points to the protruding bone fragment. [(E)—small arrowheads] Interior view of the posterior wall of the sacral canal, showing the line of fracture and [(E)—black arrow] the protruding fragment. (F) Oblique posterior view showing the imprint of the fractured bone fragment on the left meningocele.
The second patient carried his daughter on his shoulders for several hours during a hike. The exact location of leakage was not identified, but the most probable mechanism remains a tear of the dural sheath of nerve roots at an anatomically weak point in the cervical region or at the cervicothoracic junction. As the evolution was spontaneously favorable, a myelography was not performed. Neither patient had history or clinical signs of any connective tissue disorder.
The most susceptible regions for spontaneous dural leakage are the cervical and cervicothoracic junction, but the thoracic, lumbar, and sacral regions, and even the vestibular system, the cribriform plate, and the pituitary fossa, can be involved (8, 10). Once the dural tear is produced, the CSF may outflow into the extradural tissues, where it is absorbed in the initial phase after the trauma, but if the opening persists, the capacity of absorption is exceeded, CFS accumulates, and a non-absorbing membrane may gradually develop, forming a cyst (19). The time period between the formation of the fluid collection and its diagnosis varies from months to years. The volume of the CSF leak may vary from a small amount detectable only while performing the Valsalva maneuver to a large amount dispersing into the paraspinal soft tissues (5).
The clinical hallmark of SIH is the positional headache that occurs or worsens most often within the first 15 min of assuming an upright posture (20). Sometimes this delay may last even several hours. The pain is generally relieved within 30 min after lying down (20). Its onset is usually gradual with a maximum intensity reached after several minutes, but, in 15% of cases, a “thunderclap” type of headache can occur (7, 20, 21). The headache may be diffuse holocephalic, frontal, or temporal, but typically it is localized in the back of the head or base of the skull, rarely unilaterally (5, 22). It can be throbbing, and it is often characterized as a “pulling sensation” from the back of the head to the neck (22). The severity of the pain ranges from mild to incapacitating, with sometimes patients being unable to assume an upright position (5). Although orthostatic headache is typical in SIH, the postural aspect may be absent or it can diminish with the passing of time if the CSF leak remains untreated. Moreover, many different headache patterns have been described, such as exertional headaches, “second-half-of-the-day” headaches, cough headaches, and paradoxical headaches occurring or worsening when lying down. The latter is associated with the rebound of intracranial pressure after dural fistula closure (5, 23).
Apart from the headaches, other manifestations may occur in SIH, the clinical spectrum being wide and probably not yet fully defined (22, 23). The severity of the manifestations is also variable: some patients are minimally affected, while others are seriously disabled while upright (22). The most common features reported are neck pain or stiffness, nausea, and vomiting (5, 10). The stretching of cranial nerves following the downward displacement of the brain may cause, in <10% of patients, visual blurring or visual field deficits, diplopia, facial pain or numbness, facial weakness or spasm, dysgeusia, and, in 10–50% of cases, changes in hearing (“echoing” or “being underwater”), associating tinnitus, or imbalance (5, 10). If the downward displacement is important, it may lead to cerebellar tonsillar herniation, brainstem compression with depression of the vital centers, altered mental status, and coma (5, 24). Cognitive deficits ranging from minimal memory loss to signs typical of a major cognitive disorder may complicate SIH (5, 22).
In our cases, the headache was the central manifestation, there were no neurologic deficits, and only the male patient presented cervical pain.
MRI has marked a new era in the understanding of SIH and its diagnosis (7, 22). It is now considered the method of choice in the initial evaluation when SIH is suspected, being the most sensitive form of investigation. The presence and the severity of MRI abnormalities are variable, and a normal MRI, as happens in 20% of cases, does not exclude SIH (5, 22).
Typically, the brain MRI in SIH may reveal diffuse, non-nodular enhancement of the pachymeninges sparing the leptomeninges, subdural fluid collections, engorgement of venous structures, “sagging” of the brain, pituitary hyperemia, obliteration of the prepontine and perichiasmatic cisterns, and ventricular collapse (5, 7). Diffuse pachymeningeal enhancement is the first sign to appear, being identified in 73% of patients (25). The mechanisms are currently thought to be the dilatation of the dural veins and various grades of edema in the meningeal layers, external to the arachnodural junction, both secondary to the low CSF pressure (26).
The MRI findings may improve in days to weeks after effective specific treatment of a CSF leak (5). In conservatively managed cases, the enhancement may persist, but generally, it becomes less prevalent over time (27). This seems to occur not only in patients with favorable clinical evolution but also in some patients with persistent symptoms. It seems that in these cases, compensatory mechanisms manage to alleviate the effects of CSF volume depletion over time, and this results in variable improvement of the MRI signs, which does not necessarily translate to clinical improvement. CSF pressure in patients with SIH seems to increase over time, independent of the presence or absence of CT myelographically detectable CSF leakage, which also suggests that compensatory mechanisms develop over time (28).
It is not clear how soon the MRI signs appear after the onset, as the literature in this area is sparse, yet we assume it would be of the order of hours, as it would not take much longer for the dural veins to feel the effects of the low pressure. There are many published cases that document the MRI alterations in the first week (27) and we have also identified one with severe imaging findings in the first 48 h from onset of symptoms (29).
Both our patients responded to conservative measures and the follow-up imaging studies revealed significant improvements in one of the cases.
Additionally, when a CSF leak is suspected, a full spine imaging study must be performed (7). CT myelography is the investigation of choice, but non-invasive techniques, such as spinal MRI or MR myelography, are now advocated as first-line methods for the diagnosis of CSF leaks (22, 30). Spinal findings in SIH include dural enhancement, meningeal diverticula, extrathecal CSF collections, syringomyelia, and dilated epidural or intradural veins (5).
In selected cases, a lumbar puncture may also be performed. Generally, the CSF opening pressure under 60 mm H2O has diagnostic value, but CSF pressure in the normal range (7–20 cm H20) is found in many patients. Occasionally, CSF pressures may be >20 cm H20 despite an active CSF leak (28). This is the reason why the International Classification of Headache Disorders' (ICHD-3) major diagnostic criteria of intracranial hypotension headache include either low cerebrospinal fluid (CSF pressure < 60 mm H2O) or evidence of CSF leakage on imaging (31).
In our patients, lumbar puncture was performed only in the second case, as the acute nature of the pain raised the suspicion of a subarachnoid hemorrhage. The opening pressure was normal, but, as we mentioned above, this is known to occasionally occur, and it may explain the self-limited course and quick recovery (32). It is very probable that, as the epidural collection expanded, the pressure between CSF and the surrounding epidural space had balanced.
An interesting discussion arises referring to “thunderclap” headaches with normal imaging, normal CSF exam, and normal opening pressure, which, generally, remain categorized as idiopathic. This probably would have been the case in our second patient, in which cerebral signs of intracranial hypotension were faint, had we not visualized the extradural CSF collection. Our case suggests that micro-dural tears, which cannot be observed on current MRI protocols, could explain some cases of cryptogenic “thunderclap” headaches, and also that the site of the lesion may not be at the cranial level. We think it may be useful in selected cases to order a complete spinal MRI examination.
Management of intracranial hypotension depends on the severity of the signs and symptoms, on the presence of extradural CSF collections, and their size (33). Many patients (28%) respond to conservative measures, such as bed rest, adequate oral fluid intake, pain medication, caffeine, and the use of an abdominal binder (5, 8, 24, 25). However, if the CSF leak has a high flow, the symptoms may persist regardless of conservative treatment. In those cases, a non-targeted epidural blood patching is performed at the thoracolumbar junction, effective for 64% of patients (25). If unsuccessful, targeted CT-guided blood patches at the site of the leak, percutaneous placement of fibrin glue, or surgical procedures may be attempted (7, 24, 25). In Table 1, we review the treatment and outcome in recent cases of SIH (last 5 years) reported in the literature.
Generally, the prognosis is excellent for patients with brain MRI abnormalities and focal spinal CSF leaks, with most of them achieving full recovery under first-line measures. However, those with normal initial MRI findings and diffuse multilevel spinal CSF leaks may have residual altered CSF dynamics or residual CSF leaks undetectable with current imaging techniques (5).
Conclusion
SIH is a common but still underdiagnosed cause of new daily persistent headaches and may be a cause of “thunderclap” headaches. It can be spontaneous or triggered by seemingly minor traumatic events. A dural tear tends to occur at anatomically weak points, usually at the level of the cervicothoracic junction. Cerebral signs of IH must prompt an evaluation of the whole spine, as the leak may be at any level. Consideration must be given not to confuse an extradural collection with the point of the leakage, as this is not always the case. CT myelography remains the gold standard imaging exam to find the level of CSF leakage, while contrast cerebral MRI gives a solid proof of intracranial hypotension as the cause of headache. Fortunately, conservative measures are successful in most cases.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients was obtained for the publication of these cases.
Author contributions
DA-G, IB, FA, AM, AB, and DA: conceptualization. FA and CM: software and visualization. IB, DA-G, and FA: writing—original draft preparation. ST: writing—review and editing. All authors issued final approval for the version to be submitted.
Acknowledgments
3D Slicer platform (https://www.slicer.org). Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
Conflict of interest
CM was employed by MedInst Romanian-German Diagnostic Center.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schievink WI, Maya MM, Jean-Pierre S, Nuño M, Prasad RS, Moser FG, et al. Classification system of spontaneous spinal CSF leaks. Neurology. (2016) 87:673–9. doi: 10.1212/WNL.0000000000002986
2. Eross E, Dodick D, Nelson K. Orthostatic headache syndrome with CSF leak secondary to bony pathology of the cervical spine. Cephalalgia. (2002) 22:439–43. doi: 10.1046/j.1468-2982.2002.00385.x
3. Chan TLH, Cowan R, Hindiyeh N, Hashmi S, Lanzman B, Carroll I. Spinal cerebrospinal fluid leak in the context of pars interarticularis fracture. BMC Neurol. (2020) 20:1–7. doi: 10.1186/s12883-020-01740-1
4. Lohani S, Rodriguez DP, Lidov HGW, Scott RM, Proctor MR. Intrasacral meningocele in the pediatric population. J Neurosurg Pediatr. (2013) 11:615–22. doi: 10.3171/2013.3.PEDS12519
5. Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. (2006) 295:2286–96. doi: 10.1001/jama.295.19.2286
6. Grimaldi D, Mea E, Chiapparini L, Ciceri E, Nappini S, Savoiardo M, et al. Spontaneous low cerebrospinal pressure: a mini review. Neurol Sci. (2004) 25:s135–7. doi: 10.1007/s10072-004-0272-x
7. Wald JT, Diehn FE. Spontaneous intracranial hypotension. Appl Radiol. (2018) 47:18–22. doi: 10.37549/AR2510
8. Kihara T. Intracranial hypotension caused by traumatic intrasacral meningocele. J Neurol Neurosurg Psychiatry. (2004) 75:658–658. doi: 10.1136/jnnp.2003.021709
9. Kim D, Small JE. Intracranial hypotension. Neuroradiology. (2019). 158–62. doi: 10.1016/B978-0-323-44549-8.00017-1
10. Correia I, Marques IB, Ferreira R, Cordeiro M, Sousa L. Spontaneous intracranial hypotension treated with a targeted CT-guided epidural blood patch. Case Rep Med. (2016) 2016:1–5. doi: 10.1155/2016/9809017
11. Kranz PG, Gray L, Malinzak MD, Amrhein TJ. Spontaneous intracranial hypotension: pathogenesis, diagnosis, and treatment. Neuroimaging Clin N Am. (2019) 29:581–94. doi: 10.1016/j.nic.2019.07.006
12. Ferrante E, Trimboli M, Rubino F. Spontaneous intracranial hypotension: review and expert opinion. Acta Neurol Belg. (2020) 120:9–18. doi: 10.1007/s13760-019-01166-8
13. Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. (2012) 30:1323–41. doi: 10.1016/j.mri.2012.05.001
14. Bydon M, Fredrickson V, De la Garza-Ramos R, Li Y, Lehman RA, Trost GR, et al. Sacral fractures. Neurosurg Focus. (2014) 37:1–11. doi: 10.3171/2014.5.FOCUS1474
15. Rodrigues-Pinto R, Kurd MF, Schroeder GD, Kepler CK, Krieg JC, Holstein JH, et al. Sacral fractures and associated injuries. Glob Spine J. (2017) 7:609–16. doi: 10.1177/2192568217701097
17. Banno T, Ohishi T, Suzuki D, Honda Y, Kobayashi S, Matsuyama Y. Traumatic sacral pseudomeningocele with spina bifida occulta. J Neurosurg Spine. (2012) 16:78–81. doi: 10.3171/2011.8.SPINE11190
18. Rahimizadeh A, Javadi SA. Symptomatic intraspinal lumbosacral pseudomeningocele, a late consequence of root avulsion injury secondary to a gunshot wound. North Am Spine Soc J. (2020) 3. doi: 10.1016/j.xnsj.2020.100025
19. Shahinfar AH, Schechter M. Traumatic extradural cysts of the spine. Am J Roentgenol. (1966) 98:713–9. doi: 10.2214/ajr.98.3.713
20. Schievink WI. Spontaneous spinal cerebrospinal fluid leaks: a review. Neurosurg Focus. (2000) 9:1–9. doi: 10.3171/foc.2000.9.1.8
21. Ferrante E, Savino A. Thunderclap headache caused by spontaneous intracranial hypotension. Neurol Sci. (2005) 26:s155–7. doi: 10.1007/s10072-005-0433-6
22. Deline C, Schievink WI. Spontaneous Intracranial Hypotension. NORD's Rare Disease Database (2020). p. 1–9.
23. Schievink WI, Maya MM, Louy C, Moser FG, Tourje J. Diagnostic criteria for spontaneous spinal CSF leaks and intracranial hypotension. Am J Neuroradiol. (2008) 29:853–6. doi: 10.3174/ajnr.A0956
24. Greif S, Mandel S, Langer DJ, Ortiz RA. Spontaneous intracranial hypotension. Pract Neurol. (2014) 24–7.
25. D'Antona L, Jaime Merchan MA, Vassiliou A, Watkins LD, Davagnanam I, Toma AK, et al. Clinical presentation, investigation findings, and treatment outcomes of spontaneous intracranial hypotension syndrome. JAMA Neurol. (2021) 78:329–37. doi: 10.1001/jamaneurol.2020.4799
26. Farb RI, Forghani R, Lee SK, Mikulis DJ, Agid R. The venous distension sign: a diagnostic sign of intracranial hypotension at MR imaging of the brain. Am J Neuroradiol. (2007) 28:1489–93. doi: 10.3174/ajnr.A0621
27. Kranz PG, Amrhein TJ, Choudhury KR, Tanpitukpongse TP, Gray L. Time-dependent changes in dural enhancement associated with spontaneous intracranial hypotension. Am J Roentgenol. (2016) 207:1283–7. doi: 10.2214/AJR.16.16381
28. Kranz PG, Tanpitukpongse TP, Choudhury KR, Amrhein TJ, Gray L. How common is normal cerebrospinal fluid pressure in spontaneous intracranial hypotension? Cephalalgia. (2016) 36:1209–17. doi: 10.1177/0333102415623071
29. Sajjadi A, Chang I, Djalilian M, Abouzari M, Djalilian AR, Djalilian HR. Facial nerve paralysis due to spontaneous intracranial hypotension. Ear Nose Throat J. (2021) 100:NP137–8. doi: 10.1177/0145561319864577
30. Osawa I, Kozawa E, Mitsufuji T, Yamamoto T, Araki N, Inoue K, et al. Intravenous enhanced 3D FLAIR imaging to identify CSF leaks in spontaneous intracranial hypotension: comparison with MR myelography. Eur J Radiol Open. (2021) 8:1–9. doi: 10.1016/j.ejro.2021.100352
31. Olesen J. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
32. Schievink WI, Meyer FB, Atkinson JLD, Mokri B. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J Neurosurg. (1996) 84:598–605. doi: 10.3171/jns.1996.84.4.0598
33. Foley JA, Rao RD. Traumatic lumbosacral pseudomeningocele associated with spinal fracture. Spine J. (2009) 9:e5–10. doi: 10.1016/j.spinee.2009.06.018
34. Arshad M, Odell T, Fiani B, Hadi H, Johnson E, Li C, et al. Intraoperative localization and “snowman” muscle pledget repair for ventral dural defect in a case of spontaneous intracranial hypotension. Surg Neurol Int. (2022) 13:39. doi: 10.25259/SNI_1195_2021
35. Barral CM, Lemos TR, Nunes SS, Sanches SMD. The value of radionuclide cisternography in a case of spontaneous cerebrospinal leak. World J Nucl Med. (2022) 21:152–5. doi: 10.1055/s-0042-1750338
36. Casanova A, Entz L, Weinmann S, Wanke I, Reisch R. Bilateral subdural hematoma caused by spontaneous intracranial hypotension originating from a discogenic microspur successfully treated with duraplasty: a case report. Brain Spine. (2022) 2:100879. doi: 10.1016/j.bas.2022.100879
37. Choi SH, Lee YY, Kim WJ. Epidural blood patch for spontaneous intracranial hypotension with subdural hematoma: a case report and review of literature. World J Clin Cases. (2022) 10:388–96. doi: 10.12998/wjcc.v10.i1.388
38. Hughes J, Chavez B. Magnetic resonance-guided diagnosis of spontaneous intracranial hypotension in a middle-aged woman. Case Rep Neurol Med. (2022) 2022:1–4. doi: 10.1155/2022/4438923
39. Jafari E, Karaminia M, Togha M. Early and delayed rebound intracranial hypertension following epidural blood patch in a case of spontaneous intracranial hypotension. Case Rep Neurol Med. (2022) 2022:1–4. doi: 10.1155/2022/5637276
40. Kanao-Kanda M, Hiroshima S, Sato I, Nagabuchi R, Kanda H. Epidural blood patch using a racz catheter for spontaneous intracranial hypotension with unclear leak points. Cureus. (2022) 14:3–7. doi: 10.7759/cureus.23559
41. Lee SY, Park BY, Ryu T, Lee JH, Kim DH, Roh WS. Linear indices of ventricular volume on brain computed tomography as markers of effectiveness of epidural blood patch for spontaneous intracranial hypotension: a case report. Medicine. (2022) 101:e29279. doi: 10.1097/MD.0000000000029279
42. Masourou Z, Papagiannakis N, Mantzikopoulos G, Mitsikostas DD, Theodoraki K. Treating spontaneous intracranial hypotension with an anesthetic modality: the role of the epidural blood patch. Life. (2022) 12:1109. doi: 10.3390/life12081109
43. Moriyama E, Ishikawa S. Dural entry point of the vertebral artery: an overlooked route of spinal CSF leaks. NMC Case Rep J. (2022) 9:2021–265. doi: 10.2176/jns-nmc.2021-0265
44. Parra A, Relvas F, Pereira PM, Carrilho A. Spontaneous intracranial hypotension and multi-level cervical and lumbar epidural blood patches: a case report. Cureus. (2022) 14. doi: 10.7759/cureus.27721
45. Reihani H, Zarei F, Soltani A, Saeedi-Moghadam M. A notable improvement in spontaneous intracranial hypotension (SIH) after delivery in a pregnant woman: a case report. Radiol Case Reports. (2022) 17:3763–6. doi: 10.1016/j.radcr.2022.06.103
46. Shekhawat RS, Yong MH, Keong SYJ, Chen K, Too CW, Hameed S. Targeted anterior cervical epidural blood patch in a patient with spontaneous intracranial hypotension. Case Rep Neurol Med. (2022) 2022:1–5. doi: 10.1155/2022/8872775
47. Shimizu S, Ito S, Higuchi K. Multiple etiologies of secondary headaches associated with arachnoid cyst, cerebrospinal fluid hypovolemia, and nontraumatic chronic subdural hematoma in an adolescent: a case report. Surg Neurol Int. (2022) 13:386. doi: 10.25259/SNI_327_2022
48. Sobczyk P, Bojarski P, Sobstyl M. Surgical management of spontaneous intracranial hypotension syndrome: a literature review. Neurol Neurochir Pol. (2022) 50:40–3. doi: 10.5603/PJNNS.a2022.0076
49. Sugiyama A, Tamiya A, Yokota H, Mukai H, Otani R, Kuwabara S. Frontotemporal brain sagging syndrome as a treatable cause mimicking frontotemporal dementia: a case report. Case Rep Neurol. (2022) 14:82–7. doi: 10.1159/000521968
50. Tonello S, Grossi U, Trincia E, Zanus G. First-line steroid treatment for spontaneous intracranial hypotension. Eur J Neurol. (2022) 29:947–9. doi: 10.1111/ene.15195
51. Zabek MM, Turek G. Original technique of sealing cerebrospinal fluid leakage from dural sac causing spontaneous cerebral hypotension. Surg Neurol Int. (2022) 13:215. doi: 10.25259/SNI_360_2022
52. Cerulli Irelli E, Morano A, Fanella M, Giallonardo AT, Di Bonaventura C. An uncommon case of thunderclap headache in a patient with Marfan syndrome. Acta Neurol Belg. (2021) 121:1361–3. doi: 10.1007/s13760-020-01364-9
53. Cochran KT, Hasso AN, Phielipp NM. Intracranial hypotension with mild Parkinsonism and bulbar dysfunction. Neurol Clin Pract. (2021) 11:e22–4. doi: 10.1212/CPJ.0000000000000777
54. Ghosh A, Tran YX, Grant L, Numan MT, Patel R, Butler IJ. Orthostatic headaches associated with spontaneous intracranial hypotension and autonomic dysfunction—A case series in young patients. Child Neurol Open. (2021) 8:2329048X2110567. doi: 10.1177/2329048X211056709
55. Kumar BV, Sekar S, Nandana J, Erat Sreedharan S. Spontaneous intracranial hypotension and lumbosacral spondylolisthesis-case report of a rare association. Clin Neurol Neurosurg. (2021) 202:106511. doi: 10.1016/j.clineuro.2021.106511
56. Liu N, Fei Y, He F. Targeted epidural blood patch treatment for refractory spontaneous intracranial hypotension: a case report. J Neurol Surg Reports. (2021) 82:e49–52. doi: 10.1055/s-0041-1740153
57. Nisson PL, Schreck R, Graham JM, Maya MM, Schievink WI. Spontaneous intracranial hypotension secondary to congenital spinal dural ectasia and genetic mosaicism for tetrasomy 10p: illustrative case. J Neurosurg Case Lessons. (2021) 2:1–6. doi: 10.3171/CASE213
58. Pensato U, Giammello F, Baldini T, Zaniboni A, Piccolo L, Arnone G, et al. The domino effect of acephalgic spontaneous intracranial hypotension. Neurol Sci. (2021) 42:309–12. doi: 10.1007/s10072-020-04755-1
59. Podkovik S, Cavaleri J, Bullis C, Durham S. Intracranial subdural hemorrhage following closed neural tube defect repair: illustrative case. J Neurosurg Case Lessons. (2021) 2:1–5. doi: 10.3171/CASE21159
60. Jensen TSR, Rekate HL, Juhler M. Long-term telemetric intracerebral pressure monitoring as a tool in intracranial hypotension. Acta Neurochir. (2021) 163:733–7. doi: 10.1007/s00701-020-04692-0
61. Turnbull JP, Morreale VM. Spontaneous intracranial hypotension complicated by diffuse cerebral edema and episodes of severely elevated intracranial pressure: illustrative case. J Neurosurg Case Lessons. (2021) 2:2–6. doi: 10.3171/CASE21118
62. Villani LA, Digre KB, Cortez MM, Bokat C, Rassner UA, Ozudogru SN. Arachnoiditis, a complication of epidural blood patch for the treatment of low-pressure headache: a case report and systematic review. Headache J Head Face Pain. (2021) 61:244–52. doi: 10.1111/head.14076
63. Wei TT, Huang H, Chen G, He FF. Management of an intracranial hypotension patient with diplopia as the primary symptom: a case report. World J Clin Cases. (2021) 9:6544–51. doi: 10.12998/wjcc.v9.i22.6544
64. Akbar RA, Khan AA, Fernandes GM, Ahmed Mohamed AZ, Elsotouhy A, Ali YOM. Spontaneous intracranial hypotension and its management with a cervical epidural blood patch: a case report. Am J Case Rep. (2020) 21:1–5. doi: 10.12659/AJCR.925986
65. Akiba C, Bandai H, Ito Y, Maeda T, Yamaguchi K, Nakajima M, et al. Cerebrospinal fluid leak presented with the C1-C2 sign caused by spinal canal stenosis: a case report. BMC Neurol. (2020) 20:151. doi: 10.1186/s12883-020-01697-1
66. Arai S, Takai K, Taniguchi M. The algorithm for diagnosis and management of intracranial hypotension with coma: report of two cases. Surg Neurol Int. (2020) 11:267. doi: 10.25259/SNI_460_2020
67. Arumugam G, Ram S, Naidu PB, Kumaravelu S. Epidural blood patch for spontaneous intracranial hypotension (SIH): a report of two cases. Trop Doct. (2020) 50:369–73. doi: 10.1177/0049475520933140
68. Chung DJ, Liounakos J, Abrams K, Siomin V. Extreme intracranial hypotension with brain herniation treated with repeat bolus intrathecal infusions. Cureus. (2020) 12:1–7. doi: 10.7759/cureus.8089
69. Ferrante E, Trimboli M, Petrecca G, Allegrini F, Ferrante MM, Rubino F. Management of spontaneous intracranial hypotension during pregnancy: a case series. Headache J Head Face Pain. (2020) 60:1777–87. doi: 10.1111/head.13942
70. Ferrante E, Trimboli M, Pontrelli G, Rubino F. Early coma awakening after epidural blood patch. J Clin Neurosci. (2020) 71:295–6. doi: 10.1016/j.jocn.2019.08.085
71. Fuino R, Raymond S, Shapiro R. Spontaneous intracranial hypotension caused by thoracic disc disease. Headache J Head Face Pain. (2020) 60:1830–1. doi: 10.1111/head.13916
72. Hatano K, Kawamura D, Ohashi H, Hamaguchi T, Hattanmaru Y, Tani S, et al. Total spinal epidural “blood patch” application through a racz catheter in spontaneous intracranial hypotension. World Neurosurg. (2020) 135:131–4. doi: 10.1016/j.wneu.2019.11.169
73. Mandal AKJ, Ryatt A, O'Hare K, Missouris CG. Lessons of the month: HLA-B27-associated syndrome and spontaneous intracranial hypotension resulting in behavioural variant frontotemporal dementia. Clin Med. (2020) 20:e10–1. doi: 10.7861/clinmed.2020-0062
74. Mirchi A, Saint-Martin C, Myers KA. Spontaneous multilevel cerebrospinal fluid leak in marfan syndrome. Ann Neurol. (2020) 88:855–6. doi: 10.1002/ana.25837
75. Podkovik S, Kashyap S, Bonda S, Bowen I, Calayag M. Spontaneous intracranial hypotension: case study and review of the literature. Cureus. (2020) 12. doi: 10.7759/cureus.7018
76. Pollard H, Pollard R. An unusual postural headache: a case report. Chiropr Man Therap. (2020) 28:56. doi: 10.1186/s12998-020-00347-0
77. Shahab S, Soliman MAR, Alkhamees AF, Eaton S, Quint E, Im J, et al. Surgical intervention for spontaneous intracranial hypotension Type 4 CSF leak: a case report. Surg Neurol Int. (2020) 11:421. doi: 10.25259/SNI_705_2020
78. Shim HK, Park YK. Misdiagnosis of spontaneous intracranial hypotension presenting as acute mental deterioration caused by unilateral acute subdural hematoma: case report. Korean J Neurotrauma. (2020) 16:254. doi: 10.13004/kjnt.2020.16.e32
79. Uchigami H, Seki T, Hideyama T, Katsumata J, Maekawa R, Shiio Y. Spontaneous intracranial hypotension with a reversible splenial lesion after swimming. Intern Med. (2020) 59:2593–6. doi: 10.2169/internalmedicine.4971-20
80. Ueberschaer M, Patzig M, Mueller K, Schwarting J, Trabold R, Tonn JC. Case report of a cervical myelomalacia caused by a thoracolumbar intradural disc herniation leading to intracranial hypotension. J Neurol. (2020) 267:3421–4. doi: 10.1007/s00415-020-10247-1
81. Videira G, Carneiro Â, Mota Dória H, Mendes A, Andrade C. Spontaneous intracranial hypotension followed by intracranial hypertension. Neurologist. (2020) 25:109–11. doi: 10.1097/NRL.0000000000000285
82. Yamamoto A, Hattammaru Y, Uezono S. Spontaneous intracranial hypotension associated with cerebral venous thrombosis detected by a sudden seizure: a case report. JA Clin Rep. (2020) 6:59. doi: 10.1186/s40981-020-00362-3
83. Yokoi H, Chakravarthy V, Whiting B, Kilpatrick SE, Chen T, Krishnaney A. Gorham-Stout disease of the spine presenting with intracranial hypotension and cerebrospinal fluid leak: a case report and review of the literature. Surg Neurol Int. (2020) 11:466. doi: 10.25259/SNI_618_2020
84. Zou L, Li G, Zhao J, Zhang Y, Hou K. Management of spontaneous cerebrospinal fluid hypovolemia-associated massive chronic subdural hematoma with reinforced restriction of physical activity: report of three cases. J Int Med Res. (2020) 48:030006052096932. doi: 10.1177/0300060520969321
85. Bakan S, Alis D, Alis C, Kizilkilic O, Kocer N, Islak C. Reversible cerebellar herniation after epidural blood patch in a patient with spontaneous intracranial hypotension. Diagn Interv Imaging. (2019) 100:127–8. doi: 10.1016/j.diii.2018.11.005
86. Cohen-Addad DI, Efendizade A, Grigorian A, Hewitt K, Velayudhan V. Spontaneous intracranial hypotension in a patient with systemic lupus erythematosus. Radiol Case Reports. (2019) 14:1188–92. doi: 10.1016/j.radcr.2019.07.005
87. Cultrera F, Lofrese G, Nasi MT. Spontaneous intracranial hypotension due to sacral diverticula: two-case history and a pocket-sized review. Neurocirugia. (2019) 30:228–32. doi: 10.1016/j.neucir.2018.06.003
88. Fiechter M, Ott A, Beck J, Weyerbrock A, Fournier JY. Intradural non-calcified thoracic disc herniation causing spontaneous intracranial hypotension: a case report. BMC Surg. (2019) 19:66. doi: 10.1186/s12893-019-0527-3
89. Fujikawa T, Saitoh N. Female with gradual onset headache. Ann Emerg Med. (2019) 74:e13–4. doi: 10.1016/j.annemergmed.2019.02.029
90. Han S, Choi SW, Park BS, Lim JW, Kim SH, Youm JY. Cervical cerebrospinal fluid leakage concomitant with a thoracic spinal intradural arachnoid cyst. Korean J Neurotrauma. (2019) 15:214. doi: 10.13004/kjnt.2019.15.e31
91. Karsidag S, Cinar N, Sahin S, Ates MF, Tabak NA. A case of intracranial hypotension after horse riding. J Clin Neurol. (2019) 15:130. doi: 10.3988/jcn.2019.15.1.130
92. Muram S, Yavin D, DuPlessis S. Intrathecal saline infusion as an effective temporizing measure in the management of spontaneous intracranial hypotension. World Neurosurg. (2019) 125:37–41. doi: 10.1016/j.wneu.2019.01.127
93. Radhakrishnan S, Surendran D, Barathi D, Bammigatti C. Spontaneous intracranial hypotension associated with pachymeningeal enhancement in a patient with systemic lupus erythematosus (SLE): an extremely rare presenting feature. BMJ Case Rep. (2019) 12:bcr-2018-227780. doi: 10.1136/bcr-2018-227780
94. Rajesh M, Noone M, Harish Babu P. Epidural blood patch for spontaneous intracranial hypotension. Natl Med J India. (2019) 32:288. doi: 10.4103/0970-258X.295965
95. Swallow N, Doan LV. Management of presumed spontaneous intracranial hypotension. A A Pract. (2019) 12:93–5. doi: 10.1213/XAA.0000000000000852
96. Uchino H, Hamada S, Kashiwazaki D, Tomita T, Akioka N, Akai T, et al. Repeated deterioration of consciousness resulting from spontaneous intracranial hypotension associated with deep cerebral vein stagnation. World Neurosurg. (2019) 132:371–4. doi: 10.1016/j.wneu.2019.09.062
97. Williams JR, Buckley R, Oushy S, Ruzevick J, Chesnut RM. Reversible, position-dependent midbrain compression in a patient with spontaneous intracranial hypotension. World Neurosurg. (2019) 130:293–7. doi: 10.1016/j.wneu.2019.07.088
98. Camilla R, Vincenzo B, Giacomo F, Alexis Z, Antonietta S, Andrea M. Spontaneous intracranial hypotension: two steroid-responsive cases. Polish J Radiol. (2018) 83:229–33. doi: 10.5114/pjr.2018.76380
99. Chai C, Li V, Bi X. Cervical disc herniation as a rare cause of intracranial hypotension: a case report. Neurol Sci. (2018) 39:1475–7. doi: 10.1007/s10072-018-3290-9
100. Fujii N, Fujii H, Fujita A, Kim Y, Sugimoto H. Spontaneous intracranial hypotension complicated by cerebral venous thrombosis. Radiol Case Rep. (2018) 13:834–8. doi: 10.1016/j.radcr.2018.05.014
101. Girão MMV, Sousa RMP, Ribeiro MC, de O Cardoso TAM, França Júnior MC, Reis F. Spontaneous intracranial hypotension and its complications. Arq Neuropsiquiatr. (2018) 76:507–11. doi: 10.1590/0004-282x20180070
102. Lee MS, Lee S, Seo DK, Yoon SH, Choi SS. Epidural blood patch treatment of diplopia that developed after headache resolution in a patient with spontaneous intracranial hypotension. J Dent Anesth Pain Med. (2018) 18:255. doi: 10.17245/jdapm.2018.18.4.255
103. Ozyigit A, Michaelides C, Natsiopoulos K. Spontaneous intracranial hypotension presenting with frontotemporal dementia: a case report. Front Neurol. (2018) 9:1–5. doi: 10.3389/fneur.2018.00673
104. Sasikumar S, Lizarraga KJ, Gnanamanogaran B, Voisin MR, Peng P, Fasano A. Isolated gait dysfunction due to intracranial hypotension. Neurology. (2018) 91:271–3. doi: 10.1212/WNL.0000000000005953
105. Staudt MD, Pasternak SH, Sharma M, Pandey SK, Arango MF, Pelz DM, et al. Multilevel, ultra-large-volume epidural blood patch for the treatment of neurocognitive decline associated with spontaneous intracranial hypotension: case report. J Neurosurg. (2018) 129:205–10. doi: 10.3171/2017.5.JNS17249
106. Takai K, Taniguchi M. Intracranial hypotension with coma: microsurgical repair of a spinal ventral dural tear and drainage of subdural hematoma with intracranial pressure monitoring. World Neurosurg. (2018) 118:269–73. doi: 10.1016/j.wneu.2018.07.148
107. Tontisirin N, Benjhawaleemas P, Nimmaanrat S, Sathirapanya P, Laohawiriyakamol T, Tran DQ, et al. Cervical foraminal epidural blood patch for the targeted treatment of refractory cerebrospinal fluid leakage from a dural sleeve. Reg Anesth Pain Med. (2017) 43:1. doi: 10.1097/AAP.0000000000000696
Keywords: post-traumatic dural tear, CSF leakage, intracranial hypotension, sacral fracture, sacral osseous fragment, severe headache, thunderclap headache, case report
Citation: Antonescu-Ghelmez D, Butnariu I, Antonescu F, Maier C, Moraru A, Bucur AI, Anghel DN and Tuţă S (2023) Thunderclap headache revealing dural tears with symptomatic intracranial hypotension: Report of two cases. Front. Neurol. 14:1132793. doi: 10.3389/fneur.2023.1132793
Received: 27 December 2022; Accepted: 02 February 2023;
Published: 23 February 2023.
Edited by:
John K. Yue, University of California, San Francisco, United StatesReviewed by:
Jefferson W. Chen, University of California, Irvine, United StatesBharat Guthikonda, Louisiana State University Health Shreveport, United States
Copyright © 2023 Antonescu-Ghelmez, Butnariu, Antonescu, Maier, Moraru, Bucur, Anghel and Tuţă. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ioana Butnariu,  ioanalbutnariu@gmail.com
ioanalbutnariu@gmail.com
 Dana Antonescu-Ghelmez1,2
Dana Antonescu-Ghelmez1,2 Ioana Butnariu
Ioana Butnariu Adriana Moraru
Adriana Moraru Amanda Ioana Bucur
Amanda Ioana Bucur