B Cells on the Stage of Inflammation in Juvenile Idiopathic Arthritis: Leading or Supporting Actors in Disease Pathogenesis?
- 1Instituto de Medicina Molecular João Lobo Antunes, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal
- 2Rheumatology Department, Hospital de Santa Maria, Centro Hospitalar Universitário Lisboa Norte (CHULN), Lisbon Academic Medical Centre, Lisbon, Portugal
Juvenile idiopathic arthritis (JIA) is a term that collectively refers to a group of chronic childhood arthritides, which together constitute the most common rheumatic condition in children. The International League of Associations for Rheumatology (ILAR) criteria define seven categories of JIA: oligoarticular, polyarticular rheumatoid factor (RF) negative (RF-), polyarticular RF positive (RF+), systemic, enthesitis-related arthritis, psoriatic arthritis, and undifferentiated arthritis. The ILAR classification includes persistent and extended oligoarthritis as subcategories of oligoarticular JIA, but not as distinct categories. JIA is characterized by a chronic inflammatory process affecting the synovia that begins before the age of 16 and persists at least 6 weeks. If not treated, JIA can cause significant disability and loss of quality of life. Treatment of JIA is adjusted according to the severity of the disease as combinations of non-steroidal anti-inflammatory drugs (NSAIDs), synthetic and/ or biological disease modifying anti-rheumatic drugs (DMARDs). Although the disease etiology is unknown, disturbances in innate and adaptive immune responses have been implicated in JIA development. B cells may have important roles in JIA pathogenesis through autoantibody production, antigen presentation, cytokine release and/ or T cell activation. The study of B cells has not been extensively explored in JIA, but evidence from the literature suggests that B cells might have indeed a relevant role in JIA pathophysiology. The detection of autoantibodies such as antinuclear antibodies (ANA), RF and anti-citrullinated protein antibodies (ACPA) in JIA patients supports a breakdown in B cell tolerance. Furthermore, alterations in B cell subpopulations have been documented in peripheral blood and synovial fluid from JIA patients. In fact, altered B cell homeostasis, B cell differentiation and B cell hyperactivity have been described in JIA. Of note, B cell depletion therapy with rituximab has been shown to be an effective and well-tolerated treatment in children with JIA, which further supports B cell intervention in disease development.
Introduction
B cells have several important roles in autoimmunity such as autoantibody production, antigen presentation, cytokine release, and T cell activation. B cell development originates in the bone marrow, where these cells undergo different maturation stages and critical checkpoint mechanisms to ensure tolerance and are released in the periphery as immature cells. These antigen-naïve B cells circulate through the blood and lymphatic systems to secondary lymphoid organs where, upon activation and exposure to antigen, they proliferate and differentiate into memory B cells or antibody-producing plasma cells. During B cell differentiation process at germinal centers, B cells are clonally expanded and go through somatic hypermutation, affinity maturation and class or isotype switching (Figure 1) (1). Defects in central tolerance mechanisms (clonal deletion, anergy, and/ or receptor editing) occurring in the bone marrow and/ or during peripheral tolerance can contribute to the development of autoreactive B cells and autoimmune diseases (2–4). Juvenile idiopathic arthritis (JIA) is the most common rheumatic disorder in children. Disturbances in both innate and adaptive immune systems have been described in JIA patients. The study of B cells has not been extensively explored in JIA, but evidence from the literature suggests that B cells might have a relevant role in JIA pathogenesis (5–7). Notably, the detection of autoantibodies such as antinuclear antibodies (ANA), rheumatoid factor (RF), and anti-citrullinated protein antibodies (ACPA) in JIA patients supports a breakdown in B cell tolerance (8). Furthermore, it has been shown that JIA patients have increased rates of secondary V(D)J recombination (normally restricted to early B-cell precursors in the bone marrow) in peripheral blood B cells, with a skewed kappa (κ):lambda (λ) light chain usage (9–11). These data suggest that mature peripheral blood B cells of JIA patients have the potential to perform receptor revision outside the bone marrow and, therefore, promote autoimmunity (9–11). In addition, altered B cell homeostasis, B cell differentiation, and B cell hyperactivity have been described in JIA, which further supports B cell intervention in disease development (12–19).
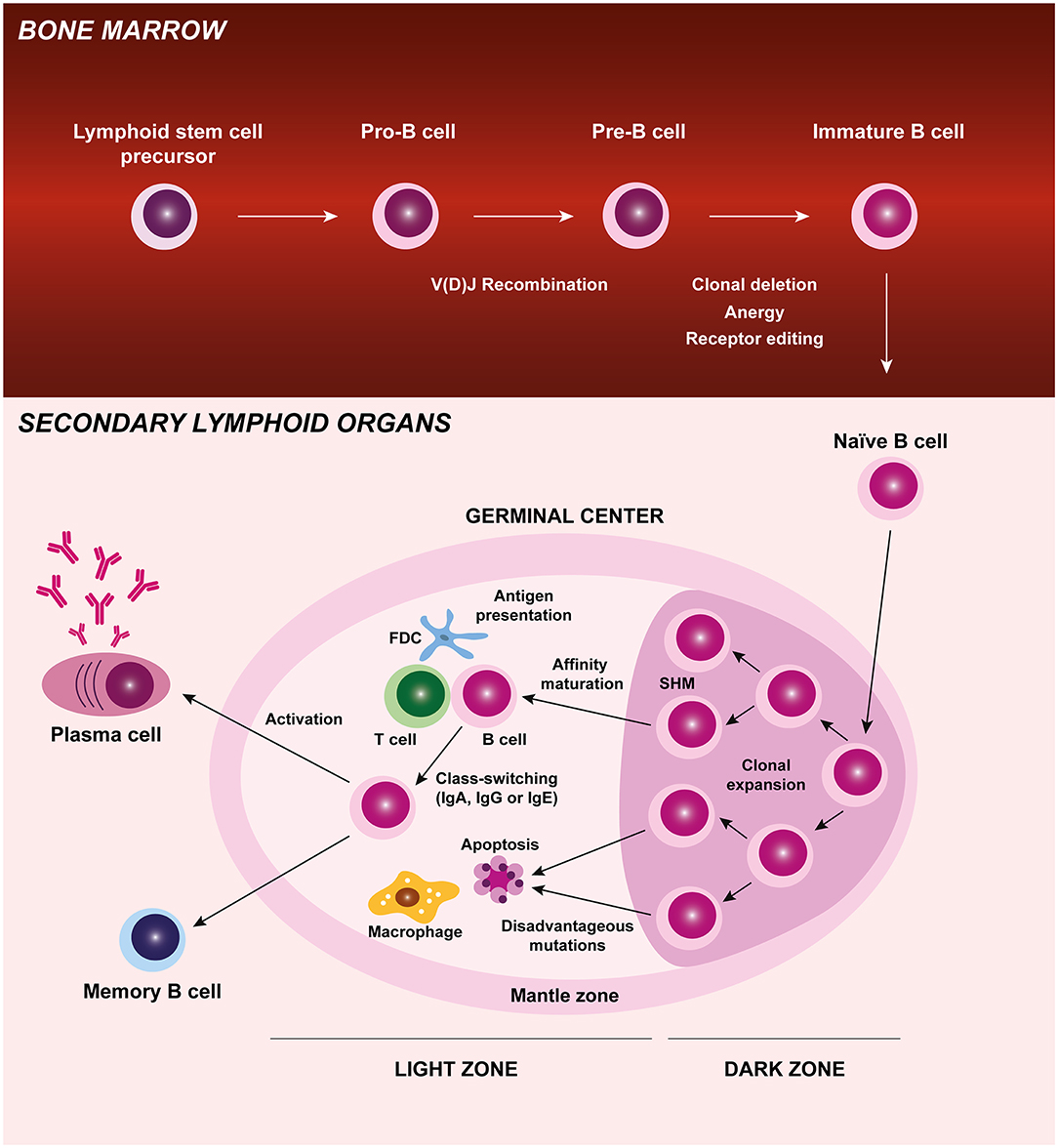
Figure 1. B cell origin and development. B cells originate from a lymphoid stem cell in the bone marrow and proceed through several maturation stages, during which V(D)J recombination and central tolerance mechanisms (clonal deletion, anergy, and receptor editing) occur. B cells leave the bone marrow and are released in the periphery as immature cells. These antigen-naïve B cells circulate through the blood and lymphatic systems to secondary lymphoid organs (lymph nodes, spleen, and Peyer's Patches) where, upon activation and exposure to antigen, they proliferate and differentiate into memory B cells or antibody-producing plasma cells. During B cell differentiation process at germinal centers, B cells are clonally expanded and go through somatic hypermutation, affinity maturation, and class-switching. Autoreactive B cells that may develop during B cell differentiation process and recognize self-antigens are eliminated by apoptosis. FDC, follicular dendritic cell; Ig, immunoglobulin; SHM, somatic hypermutation.
Juvenile Idiopathic Arthritis: Definition, Classification and Disease Categories
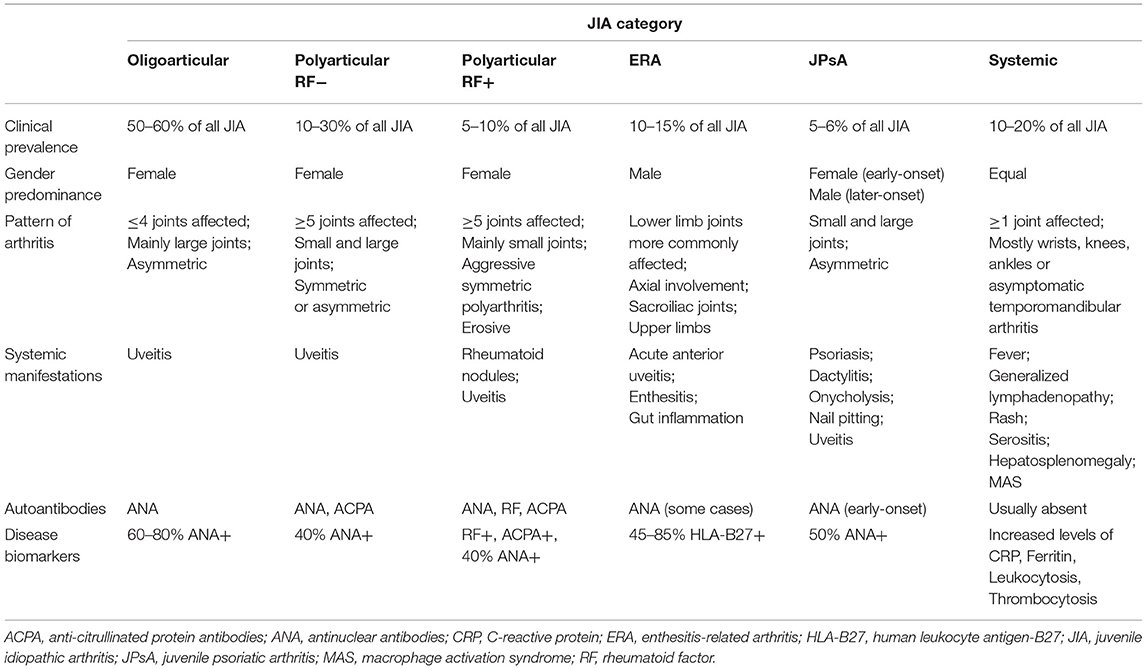
Juvenile idiopathic arthritis (JIA) is a term used to classify a group of heterogeneous chronic childhood inflammatory arthritides of unknown etiology, which together constitute the most common rheumatic condition in children (20). JIA is characterized by a chronic inflammatory process affecting the synovia that begins before the age of 16 and persists at least 6 weeks. Nonetheless, in some children, JIA can be a lifelong condition. JIA affects not only joints, but also extra-articular structures, including eyes, skin, and internal organs and, if not treated, can lead to serious disability and loss of quality of life (21, 22). The International League of Associations for Rheumatology (ILAR) criteria define seven categories of JIA: oligoarticular, polyarticular RF negative (RF-), polyarticular RF positive (RF+), systemic, enthesitis-related arthritis, psoriatic arthritis, and undifferentiated arthritis (23). The ILAR classification includes persistent and extended oligoarthritis as subcategories of oligoarticular JIA, but not as distinct categories. JIA initial classification is determined according to the clinical features presented during the first 6 months of disease course, such as the number of affected joints, severity of disease, and presence or absence of inflammation in other parts of the body. The onset of new clinical features during the course of the disease determines the final disease subtype.
Oligoarticular
Oligoarticular JIA (oJIA) is the most common JIA subtype. It is defined as asymmetric arthritis affecting up to four joints, mainly large joints such as the knee, ankle, wrist, and/ or elbow, during the first 6 months after disease onset (23). oJIA can be subcategorized in two types of arthritis: persistent oJIA (no more than four joints are affected during the course of the disease) and extended oJIA (more than four joints are affected after the first 6 months of disease). oJIA usually begins before 4 years of age and female gender is predominantly affected (24). ANA are present in up to 60–80% of patients with oJIA. Importantly, oJIA is associated with a high risk of uveitis (24, 25), particularly in ANA positive (ANA+) patients (26–28).
Polyarticular
Polyarticular JIA (pJIA) is the second most common JIA subtype, defined as arthritis in which 5 or more joints are affected during the first 6 months of the disease. There are two forms of pJIA: polyarticular RF negative (pJIA RF-) and polyarticular RF positive (pJIA RF+) (23). Female gender is predominant in both types of pJIA.
Polyarticular Rheumatoid Factor Negative
Patients with pJIA RF- have mostly asymmetric arthritis of small and large joints, and disease onset usually occurs between 2 and 12 years old. ANA can be detected in up to 40% of pJIA patients and uveitis can be present in up to 10% of cases (23–25).
Polyarticular Rheumatoid Factor Positive
Patients with pJIA RF+ have symmetric arthritis mainly of small joints (metacarpophalangeal joints and wrists) and disease onset is more frequent in late childhood or adolescence. Arthritis development is associated to a more erosive and aggressive disease progression (23–25).
Systemic
Systemic JIA (sJIA) is a disease subtype defined by the presence of arthritis in one or more joints and concomitant systemic manifestations that include fever persisting for more than 2 weeks, generalized lymphadenopathy, rash, hepatosplenomegaly, and/ or serositis (23). Disease onset may occur at any time during childhood and both female and male genders are equally affected. Autoantibodies are usually absent (25, 29). A dysregulation of the innate immune system has been associated to the systemic inflammation present in sJIA, suggesting that this JIA subtype may rather be part of the spectrum of autoinflammatory disorders (29–33). Nevertheless, alterations in adaptive immunity have also been described in sJIA (34–38).
Enthesitis-Related Arthritis
Enthesitis-related arthritis (ERA) is a subtype of JIA that affects the joints of the lower limbs (hip, knee, ankle, and foot) in association with enthesitis. In addition, axial involvement and arthritis of the sacroiliac joints and upper limbs, particularly the shoulders, can also occur (23). ERA is more frequent in male gender. Disease onset usually occurs in late childhood or adolescence. Acute anterior uveitis and gut inflammation can also be present in ERA patients. The diagnosis of this JIA subtype is strongly associated to the major histocompatibility complex (MHC) class I antigen human leukocyte antigen (HLA)-B27 (25).
Psoriatic Arthritis
Juvenile psoriatic arthritis (JPsA) is characterized by an asymmetric arthritis of small and large joints and the presence either of a psoriatic rash or, in the absence of rash, at least two of the following criteria: first-degree relative with psoriasis, nail pitting or onycholysis, and dactylitis (23). Clinical symptoms may also include uveitis. JPsA is composed of two subgroups, differentiated by age at onset. Children with early-onset JPsA (<6 years old) are predominantly female, ANA+, more predisposed to uveitis, with arthritis of the wrists and small joints of the hands and feet. In contrast, children with later-onset JPsA are more associated to male gender, axial disease, enthesitis, and HLA-B27 positivity (39, 40).
Undifferentiated Arthritis
Undifferentiated juvenile idiopathic arthritis includes patients who do not fulfill the criteria for any JIA category above described, or who meet the criteria for more than one (23, 41).
The main clinical features of all JIA categories are summarized in Table 1. Notably, the complexity and heterogeneity of JIA diagnosis is still controversial and subject to new classification proposals (42, 43).
Etiology and Risk Factors of Juvenile Idiopathic Arthritis
The cause of JIA is unknown. Nevertheless, JIA has been established as an autoimmune disorder in which genetic susceptibility and environmental factors are associated to disease development. JIA might be initially triggered by the exposure to environmental factors in children with a genetic predisposition to synovial inflammation. Infections, vaccines, antibiotics, vitamin D deficiency, stress, and trauma have been proposed as environmental risk factors for JIA progression (25, 44, 45). In fact, it has been reported that infectious viruses (Epstein-Barr virus, Parvovirus B, Rubivirus, and Hepatitis B virus) and bacteria (Salmonella spp., Shigella spp., Campylobacter spp., Streptococcus pyogenes, Bartonella henselae, Mycoplasma pneumoniae, and Chlamydophila pneumonia) may act as triggering agents of JIA (46). Furthermore, disturbances in the gut microbiome have been shown to increase the risk of JIA development (47–49). Additionally, evidence from the literature suggests that maternal smoking during pregnancy can also be a risk factor for JIA (44, 50). Genetic predisposition to JIA is mainly due to human leukocyte antigen (HLA) class II molecules, although HLA class I molecules and non-HLA genes have also been implicated, depending on the disease category (24, 25, 29, 51–57).
Immunopathogenesis of Juvenile Idiopathic Arthritis
The mechanisms of immunopathogenesis of JIA are still poorly understood. JIA categories are complex, heterogeneous, with different contributions of immune system players and effector cells (24, 25, 58–63). Indeed, several studies have demonstrated a predominance of adaptive immunity in the pathogenesis of oJIA, pJIA, ERA, and JPsA (14, 17, 18, 24, 25, 39, 57, 64–86), whereas innate immune responses are the major contributors to disease development and progression in sJIA (29, 59, 87–98). In fact, oJIA, pJIA, ERA, and JPsA are classified as autoimmune diseases, while sJIA has been proposed as an autoinflammatory disorder (25, 58, 59). In JIA, joint inflammation, swelling and tissue destruction are a hallmark of the disease. The pathophysiology mechanisms associated to JIA development are related to an abnormal activation of immune system cells such as B cells, T cells, natural killer (NK) cells, dendritic cells (DCs), monocytes, neutrophils, plasma cells, and to the production and release of pro-inflammatory mediators (cytokines, chemokines, enzymes such as matrix metalloproteinases, aggrecanases, and cathepsins) that ultimately lead to cartilage and bone destruction and systemic manifestations. The inflammatory process that occurs at the synovial joint leads to the thickening of the synovial membrane due to an excessive proliferation of synoviocytes and infiltration of the sub-lining layer of the synovium by immunocompetent cells (lymphocytes, macrophages, granulocytes, plasma cells…), which causes hyperplasia and hypertrophy of the synovium (Figure 2). Consequently, intra-articular hypoxia occurs and pathological angiogenesis initiates. The new blood vessels that form within the synovium increase blood supply and contribute to the migration of pro-inflammatory cells into the joint, thus forming a pathological synovium known as “pannus.” Overall, the complex cellular networks and the release of inflammatory mediators that occur within JIA synovium stimulate chondrocytes and osteoclasts that trigger cartilage and bone erosion, respectively (Figure 2) (99–107).
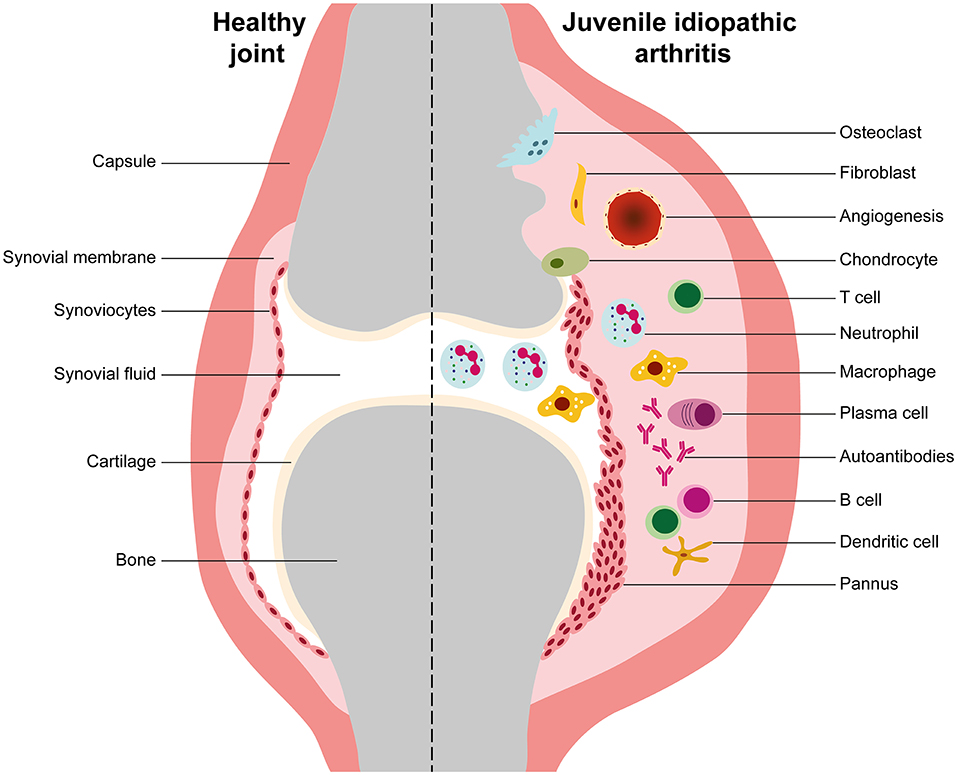
Figure 2. Synovial inflammation in juvenile idiopathic arthritis. This is a representative scheme illustrating the differences between healthy and juvenile idiopathic arthritis (JIA) joints. In JIA, the inflammatory process that occurs at the synovial joint is characterized by an uncontrolled proliferation of synoviocytes and cellular infiltration of the sub-lining layer of the synovium by macrophages, granulocytes, B cells, T cells, and plasma cells, which causes synovial membrane hyperplasia. Consequently, intra-articular hypoxia occurs and pathological angiogenesis initiates. The new blood vessels that form within the synovium increase blood supply and contribute to the migration of pro-inflammatory cells into the joint, thus forming a pathological synovium known as “pannus.” The complex cellular networks that occur within JIA synovium and the production of inflammatory mediators such as cytokines, chemokines and metalloproteinases stimulate chondrocytes and osteoclasts that trigger cartilage and bone erosion, respectively.
B Cell Roles in Juvenile Idiopathic Arthritis Pathophysiology
JIA has been classically considered a T-cell driven autoimmune disease, except for sJIA subtype, in which innate immune cells have a central role in disease pathogenesis as previously mentioned. However, the detection of autoantibodies reacting with different target antigens in JIA patients suggests a central role of B cells in JIA pathophysiology. In fact, B cells may have important roles in JIA pathogenesis through autoantibody production, antigen presentation, cytokine release, and/ or T cell activation (Figure 3) (5–7). Although the study of B cells has not been extensively explored in JIA, evidence from the literature suggest the occurrence of alterations in B cell differentiation, homeostasis and hyperactivity in JIA patients, which can contribute to disease progression.
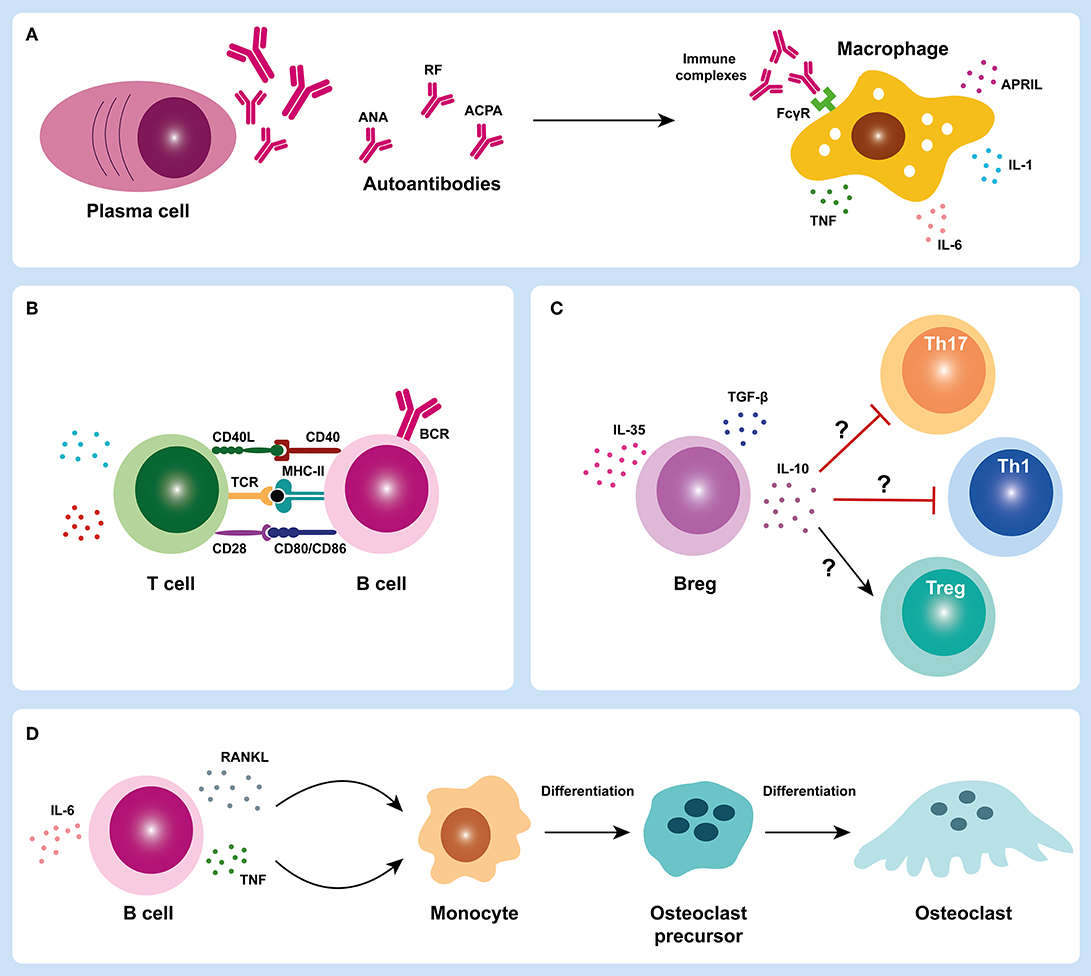
Figure 3. B cell roles in juvenile idiopathic arthritis. B cells can have several important roles in juvenile idiopathic arthritis (JIA) pathogenesis through autoantibody production (A), antigen presentation and/ or T cell activation (B,C), cytokine release and contribute to bone damage in JIA synovial joint (D). In JIA, activated B cells can differentiate into autoantibody-producing plasma cells. Plasma cells produce autoantibodies that can form immune complexes that deposit in the joints and trigger macrophage activation through Fc-gamma receptors (FcγR) (A). Activated macrophages can release several pro-inflammatory mediators such as cytokines (IL-1, IL-6, and TNF) that contribute to the inflammatory process (A). B cells can also act as efficient antigen presenting cells and activate T cells (B). Regulatory B cells (Bregs) may also have an important contribution in JIA pathogenesis through either a defective suppression of T helper cell subsets (Th1 and Th17) and/ or an impairment of regulatory T cells (Tregs) activation (C). B cells can also release cytokines such as TNF and RANKL that can activate osteoclastogenesis and trigger bone erosion in JIA synovial joint (D). ACPA, anti-citrullinated protein antibodies; ANA, antinuclear antibodies; APRIL, A proliferation-inducing ligand; BCR, B cell receptor; Breg, regulatory B cell; CD, cluster of differentiation; FcγR, Fc-gamma receptor; IL, interleukin; MHC-II, major histocompatibility complex class II; RANKL, receptor activator of nuclear factor kappa-B ligand; RF, rheumatoid factor; TCR, T cell receptor; TGF-β, transforming growth factor beta; Th, T helper; TNF, tumor necrosis factor; Treg, regulatory T cell.
Autoantibody Production
Autoantibody production is a hallmark function of B cells in autoimmunity. In JIA, autoantibodies such as ANA, RF and ACPA can be detected in the serum of these patients (8, 108–114), which supports a breakdown in B cell tolerance. ANA are autoantibodies that can target several autoantigens within cell nucleus structures including nucleic acids, nucleosomes, phospholipids, and several nuclear and nucleolar proteins (115). These autoantigens, which are normally “hidden,” are exposed to antigen presenting cells during cell death, particularly during apoptosis. ANA can be detected in several autoimmune diseases such as systemic lupus erythematosus (SLE), RA, Sjögren's syndrome, idiopathic thrombocytopenic purpura, mixed connective tissue disease, juvenile dermatomyositis, autoimmune hepatitis, primary biliary cirrhosis, ulcerative colitis, and autoimmune thyroiditis (116–125). In JIA patients, the overall seroprevalence of ANA among all subtypes of JIA combined is < 50% (126). ANA are more commonly detected in oJIA and pJIA (mostly in pJIA RF-) patients and are particularly more prevalent in young, female oJIA patients (127). In JPsA patients, ANA positivity is also more strongly associated with early-onset disease and female predominance (39). ANA are less commonly detected in sJIA and undifferentiated JIA (126). Although the exact contribution of ANA to JIA pathology remains unclear, previous reports have suggested that ANA positivity is associated with the development of ectopic lymphoid structures in synovial tissue (16). Interestingly, the presence of a lymphoid organization in synovial tissue from ANA+ JIA patients was strongly related to the concomitant degree of plasma cells infiltration (16). Thus, the development of these lymphoid structures could contribute to the interactions between autoreactive B and T cells, which could directly support the production of these autoantibodies and the inflammatory process in the joint. Furthermore, it has been demonstrated that ANA are associated to a higher risk of uveitis (119, 128). RF is an immunoglobulin of any isotype (predominantly IgM) that specifically recognizes the Fc portion of IgG molecules, which was first described in RA patients (129, 130) and is currently included in the classification criteria for RA (131). RF can be detected in other autoimmune disorders such as SLE, Sjögren's syndrome, systemic sclerosis, mixed connective tissue disease, polymyositis, dermatomyositis, as well as in healthy individuals (132, 133). RF have also been identified in JIA patients (8, 134) and, despite being present in a small subgroup of pJIA patients (only 5% of total JIA patients), RF positivity is associated with a worse disease prognosis (113, 135). Indeed, patients with pJIA RF+ are at higher risk of a more aggressive disease course with cartilage and bone erosions than JIA patients without RF (113, 136–138). Notably, pJIA RF+ is considered the pediatric version of adult RA (139, 140). In fact, it has been demonstrated that the majority of pJIA patients, particularly pJIA RF+, evolve to RA in adulthood (21). Of note, these observations reinforce that pJIA RF+ and RA are likely to have similar underlying pathological mechanisms and that current treatment strategies applied in RA are directly relevant to pJIA RF+. RF can have important physiological roles in the normal immune system such as promoting phagocytosis and the removal of antigen-antibody complexes in the course of infection; fixation of complement; and enhancing B cell antigen uptake and presentation to CD4+ T cells (141). Nevertheless, these naturally-occurring RF are generally low-affinity and polyreactive, whereas pathogenic RF tend to have undergone affinity maturation (133, 142). Although the mechanisms of RF production are not entirely understood, previous reports have described to be dependent on immune-complex recognition by B cell receptors in the context of toll-like receptor stimulation, as well as T cell help (143–145). ACPA are autoantibodies that recognize citrullinated peptides and proteins and, similarly to RF, are included in the classification criteria for RA (131). Although ACPA are highly specific to RA diagnosis, these antibodies can also be detected in JIA patients, particularly in pJIA RF+ (111, 112, 114, 146–148). In fact, it has been demonstrated that ACPA detected in pJIA RF+ patients express the inherently autoreactive 9G4 idiotope, which supports an activation of autoreactive 9G4+ B cells in JIA (148), similarly to what has been described in RA patients (149). Importantly, ACPA detection in JIA patients is associated to more severe and erosive disease progression, which might implicate an earlier and more intensive treatment (8, 112–114, 146, 150, 151). During inflammation, ACPA might be produced in a process called citrullination, a post-translational modification catalyzed by peptidylarginine deiminases (PAD) enzymes in which arginine amino acid residues are enzymatically converted into citrulline residues in a wide range of proteins (152, 153). These structural changes in proteins can form new epitopes that trigger ACPA production by autoreactive B cells. Of note, both ACPA and RF autoantibodies are able to form immune complexes that deposit in the joints. These immune complexes can activate complement and macrophages through Fc-gamma receptors (FcγR) and induce cytokine release that contribute to the inflammatory process in JIA (Figure 3A) (8, 154, 155). Importantly, evidence of hypergammaglobulinaemia correlated with clinical disease activity has been described in JIA patients (mainly in oJIA and pJIA), which is consistent with B cell hyperactivity (14).
Antigen Presentation, Cytokine Release, and T Cell Activation
During the inflammatory process in JIA, B cells can function as efficient antigen presenting cells and, once activated, can release cytokines and stimulate T cell activation, thus contributing to the exacerbation of inflammation (Figure 3B). A distinctive feature of chronic inflammatory arthritis is the presence of synovial lymphocytic infiltrates that play a role in disease pathogenesis by secretion of pro-inflammatory cytokines and other soluble mediators (156–166). Indeed, both B and T cells are detected in synovial infiltrates from JIA and RA patients (17, 18, 69, 79, 156, 163–165, 167–172). In particular, high levels of plasma cells infiltration have been detected in the synovial membrane of JIA patients (69, 173). Furthermore, it has been shown that lymphoid neogenesis in JIA is correlated to ANA positivity and plasma cells infiltration not only in synovia, but also in iris tissue, which further supports a dysregulated B cell activation in JIA patients (16, 174, 175). Notably, it was shown that JIA patients with ANA+ anterior uveitis often show an infiltrate of plasma cells in iris (174). In addition, it has been demonstrated that activated memory B cells accumulate in the inflamed joints of patients with JIA (17, 18, 171, 176). Of note, it was shown that class-switched CD27+ and CD27- memory B cells expressed up-regulated levels of the costimulatory molecules CD80 and CD86 and could activate allogenic CD4+ T cells in vitro more effectively when compared to peripheral blood B cells (18). Additionally, it was observed that synovial B cells could not only induce the activation and polarization of T helper (Th)-1 cells, but also secrete Th1-polarizing cytokines (18). Overall, the accumulation of memory B cells in the synovia of JIA patients suggests antigen-driven activation of B cells within the inflamed tissues potentially triggered by local antigens (18). Changes in B cell subpopulations have also been documented in peripheral blood and synovial fluid from JIA patients (12–14, 17, 18, 92, 177–179). Indeed, expansions of CD5+ B cells and transitional (CD24highCD38high) B cells have been reported in oJIA and pJIA patients (12–14, 17), which suggest that defects in B cell differentiation and homeostasis may occur in JIA. Of note, CD5+ B cells might be involved in autoantibody production and have the ability to function as antigen presenting cells (180–182). Moreover, it has been demonstrated that transitional B cells (CD24highCD38high) can secrete interleukin (IL)-10 and regulate CD4+ T cell proliferation and differentiation toward T helper effector cells (183–186). Furthermore, transitional B cells can also secrete pro-inflammatory cytokines such as IL-6 and tumor necrosis factor (TNF) that contribute to disease pathogenesis (187–189). In addition, alterations in regulatory B cells (Bregs) have also been described in JIA patients that might contribute to disease development (178, 190). Indeed, it was shown that the frequency of CD19+CD24highCD38high Bregs was significantly decreased in peripheral blood and synovial fluid from JIA patients (178). Interestingly, patients with pJIA RF+ had reduced levels of CD19+CD24highCD38high Bregs when compared to patients with pJIA RF- (178). Moreover, the frequency of IL-10-producing Bregs (B10 cells) was significantly lower in active JIA patients in comparison to inactive patients (178). In fact, alterations in regulatory B cell numbers and/ or functions have been described in several autoimmune diseases (183, 191–195). Previous studies have shown that RA patients have decreased frequencies of CD19+CD24highCD38high Bregs in circulation and that these cells fail to suppress Th17 cells differentiation (183). Furthermore, RA patients with active disease have reduced levels of CD19+CD24highCD38high Bregs in peripheral blood when compared to patients with inactive disease, similarly to what has been described in JIA (183). Taken together, these observations suggest that patients with JIA might have altered regulatory B cell functions, with defective suppression of T helper cell subsets (Th1 and Th17) and/ or impaired regulatory T cells activation, as previously described in other autoimmune diseases (Figure 3C) (183, 191–195). In addition, increased levels of cytokines relevant for B cell activation, maturation, differentiation and survival such as B cell activating factor (BAFF), A proliferation-inducing ligand (APRIL), IL-6 or IL-21, have been detected in serum and/ or synovial fluid from JIA patients (76, 89, 196–201). Of note, BAFF and APRIL serum levels from JIA patients were significantly correlated with disease activity (199). Furthermore, elevated peripheral blood BAFF mRNA levels have been described in JIA patients (202). Also, increased levels of IL-6 and IL-21, cytokines relevant for B cell maturation and plasma cell differentiation, respectively, have been found in synovial fluid from JIA patients (76). Interestingly, IL-21 synovial fluid concentration was particularly increased in pJIA patients (76). Moreover, it was observed that a worse disease severity at baseline in JIA patients was associated with increased IL-6 plasma levels (201). Overall, these observations support the occurrence of B cell triggering mechanisms in JIA that contribute to disease progression. B cells can also act as major producers of receptor activator of nuclear factor kappa-B ligand (RANKL), a key cytokine in osteoclastogenesis, and bone erosion (167, 169, 203). Interestingly, it has been shown that JIA patients have significantly increased levels of RANKL not only in serum, but also in synovial fluid (99, 204–206). Importantly, higher levels of RANKL were associated with a more serious disease, particularly in pJIA patients (99, 205, 206). Thus, these observations suggest that B cells might have a critical role in bone damage and joint destruction in JIA (Figure 3D).
Treatment and B Cell Targeted Therapies in Juvenile Idiopathic Arthritis
Treatment of JIA is adjusted according to the severity of the disease as combinations of non-steroidal anti-inflammatory drugs (NSAIDs), synthetic and/ or biological disease modifying anti-rheumatic drugs (DMARDs) (207–210). Intra-articular corticosteroid injections can also be used with great effectiveness (211, 212). Systemic administration of corticosteroids can have a positive short-term effect, but its prolonged administration is associated with severe side effects such as osteoporosis, growth suppression or immunosuppression (213–215). The American College of Rheumatology (ACR) recommends early use of DMARDs in JIA patients, specifically methotrexate (MTX) (214, 216–218). Furthermore, biological drugs have also been approved for JIA treatment, including TNF inhibitors (etanercept, infliximab, adalimumab, and golimumab) (219–223); the T-cell modulator abatacept (224, 225); the humanized monoclonal antibody against the IL-6 receptor (IL-6R) tocilizumab (226–228) and the IL-1 inhibitor canakinumab (for sJIA) (229–231). Moreover, IL-1R antagonist anakinra (232, 233) and IL-1 inhibitor rilonacept (234, 235) can also be used as effective treatments in sJIA. Additionally, the Janus kinase inhibitor (JAKi) tofacitinib is already approved for the treatment of JIA and clinical trials are currently ongoing to assess the effectiveness and safety of baricitinib in JIA treatment, with preliminary data showing promising results (236–240). Despite the progress achieved in the last years in JIA treatment, about half of the patients continue to require active treatment into adult life, whereas complete remission is reached in only 20–25% of patients (241, 242). B cell depletion therapy with rituximab, a monoclonal antibody that targets CD20 expressed on B cells, is a successful treatment in autoimmune diseases such as RA (243, 244). Nevertheless, few studies have investigated the effectiveness and safety of this treatment option in JIA (7, 245–253). Rituximab is currently only considered in JIA patients refractory to first-line treatments such as TNF inhibitors and standard immunosuppressive therapies, namely MTX (7, 25, 248, 253). Indeed, it has been demonstrated that rituximab is an effective therapeutic option in patients with severe forms of oligoarticular, polyarticular, and systemic JIA, refractory to several prior agents (245–248, 251–253). Adverse events such as infusion reactions, hypogammaglobulinaemia and infections have been reported in pediatric patients after B cell depletion therapy and must be taken into account (254, 255). Nonetheless, rituximab has been reported as an effective and well-tolerated treatment in children, with a low rate of serious infections described in JIA patients, although it is not formally approved by the European Medicines Agency (EMA) for this indication (253). Notably, the efficacy of rituximab treatment in JIA patients strongly supports that B cells play an important role in JIA pathogenesis.
Conclusions
JIA is the most common rheumatic disorder in children, classified in seven different categories according to ILAR criteria. JIA can cause significant disability and loss of quality of life, if not treated. Disturbances in innate and adaptive immune responses have been implicated in JIA development. B cells are important players in autoimmune diseases and may have roles in JIA pathogenesis through autoantibody production, antigen presentation, cytokine release, and/ or T cell activation. The study of B cells has not been extensively explored in JIA, but evidence from the literature suggests that B cells might have indeed a relevant role in JIA pathophysiology. In fact, the detection of autoantibodies such as ANA, RF and ACPA in JIA patients supports a breakdown in B cell tolerance. Furthermore, altered B cell homeostasis, B cell differentiation, and B cell hyperactivity have been described in JIA, which further supports B cell intervention in disease development. B cell depletion therapy with rituximab is a treatment option considered in JIA patients refractory to first-line biologic treatments such as TNF inhibitors, which has been shown to be an effective and well-tolerated treatment in children with JIA, supporting B cell involvement in JIA pathogenesis. Therefore, further research studies concerning the role of B cells in JIA pathophysiology should be explored, which might be relevant for a better knowledge of disease pathogenesis and have important implications in current and future B-cell targeted therapeutic approaches in JIA.
Author Contributions
RAM conceptualized the manuscript, reviewed the literature, and wrote the manuscript. JEF revised the manuscript and contributed with important intellectual input. All authors read and approved the final manuscript.
Funding
The authors would like to acknowledge Sociedade Portuguesa de Reumatologia (SPR) for funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Moura R, Agua-Doce A, Weinmann P, Graça L, Fonseca JE. B cells from the bench to the clinical practice. Acta Reumatol Port. (2008) 33:137–54.
2. Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. (2008) 20:632–8. doi: 10.1016/j.coi.2008.09.001
3. von Boehmer H, Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol. (2010) 11:14–20. doi: 10.1038/ni.1794
4. Reijm S, Kissel T, Toes REM. Checkpoints controlling the induction of B cell mediated autoimmunity in human autoimmune diseases. Eur J Immunol. (2020) 50:1885–94. doi: 10.1002/eji.202048820
5. Morbach H, Girschick HJ. Do B cells play a role in the pathogenesis of juvenile idiopathic arthritis? Autoimmunity. (2009) 42:373–5. doi: 10.1080/08916930902832306
6. Wiegering V, Girschick HJ, Morbach H. B-cell pathology in juvenile idiopathic arthritis. Arthritis. (2010) 2010:759868. doi: 10.1155/2010/759868
7. Wilkinson MGL, Rosser EC. B cells as a therapeutic target in paediatric rheumatic disease. Front Immunol. (2019) 10:214. doi: 10.3389/fimmu.2019.00214
8. Mahmud SA, Binstadt BA. Autoantibodies in the pathogenesis, diagnosis, and prognosis of juvenile idiopathic arthritis. Front Immunol. (2018) 9:3168. doi: 10.3389/fimmu.2018.03168
9. Faber C, Morbach H, Singh SK, Girschick HJ. Differential expression patterns of recombination-activating genes in individual mature B cells in juvenile idiopathic arthritis. Ann Rheum Dis. (2006) 65:1351–6. doi: 10.1136/ard.2005.047878
10. Low JM, Chauhan AK, Moore TL. Abnormal kappa:lambda light chain ratio in circulating immune complexes as a marker for B cell activity in juvenile idiopathic arthritis. Scand J Immunol. (2007) 65:76–83. doi: 10.1111/j.1365-3083.2006.01859.x
11. Morbach H, Richl P, Faber C, Singh SK, Girschick HJ. The kappa immunoglobulin light chain repertoire of peripheral blood B cells in patients with juvenile rheumatoid arthritis. Mol Immunol. (2008) 45:3840–6. doi: 10.1016/j.molimm.2008.05.011
12. Martini A, Massa M, De Benedetti F, Viola S, Neirotti G, Burgio RG. CD5 positive B lymphocytes in seronegative juvenile arthritis. J Rheumatol. (1990) 17:932–5.
13. Jarvis JN, Kaplan J, Fine N. Increase in CD5+ B cells in juvenile rheumatoid arthritis. Relationship to IgM rheumatoid factor expression and disease activity. Arthritis Rheum. (1992) 35:204–7. doi: 10.1002/art.1780350213
14. Wouters CHP, Ceuppens JL, Stevens EaM. Different circulating lymphocyte profiles in patients with different subtypes of juvenile idiopathic arthritis. Clin Exp Rheumatol. (2002) 20:239–48.
15. Lepore L, Del Santo M, Malorgio C, Presani G, Perticarari S, Prodan M, et al. Treatment of juvenile idiopathic arthritis with intra-articular triamcinolone hexacetonide: evaluation of clinical effectiveness correlated with circulating ANA and T gamma/delta + and B CD5+ lymphocyte populations of synovial fluid. Clin Exp Rheumatol. (2002) 20:719–22.
16. Gregorio A, Gambini C, Gerloni V, Parafioriti A, Sormani MP, Gregorio S, et al. Lymphoid neogenesis in juvenile idiopathic arthritis correlates with ANA positivity and plasma cells infiltration. Rheumatology. (2007) 46:308–13. doi: 10.1093/rheumatology/kel225
17. Corcione A, Ferlito F, Gattorno M, Gregorio A, Pistorio A, Gastaldi R, et al. Phenotypic and functional characterization of switch memory B cells from patients with oligoarticular juvenile idiopathic arthritis. Arthritis Res Ther. (2009) 11:R150. doi: 10.1186/ar2824
18. Morbach H, Wiegering V, Richl P, Schwarz T, Suffa N, Eichhorn E-M, et al. Activated memory B cells may function as antigen-presenting cells in the joints of children with juvenile idiopathic arthritis. Arthritis Rheum. (2011) 63:3458–66. doi: 10.1002/art.30569
19. Zahran AM, Abdallah AM, Saad K, Osman NS, Youssef MAM, Abdel-Raheem YF, et al. Peripheral blood B and T cell profiles in children with active juvenile idiopathic arthritis. Arch Immunol Ther Exp. (2019) 67:427–32. doi: 10.1007/s00005-019-00560-7
20. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. (2007) 369:767–78. doi: 10.1016/S0140-6736(07)60363-8
21. Oliveira-Ramos F, Eusébio MM, Martins F, Mourão AF, Furtado C, Campanilho-Marques R, et al. Juvenile idiopathic arthritis in adulthood: fulfilment of classification criteria for adult rheumatic diseases, long-term outcomes and predictors of inactive disease, functional status and damage RMD. Open. (2016) 2:e000304. doi: 10.1136/rmdopen-2016-000304
22. Oliveira Ramos F, Rodrigues A, Magalhaes Martins F, Melo AT, Aguiar F, Brites L, et al. Health-related quality of life and disability in adults with juvenile idiopathic arthritis: comparison with adult-onset rheumatic diseases. RMD Open. (2021) 7:e001766. doi: 10.1136/rmdopen-2021-001766
23. Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. (2004) 31:390–2.
24. Macaubas C, Nguyen K, Milojevic D, Park JL, Mellins ED. Oligoarticular and polyarticular JIA: epidemiology and pathogenesis. Nat Rev Rheumatol. (2009) 5:616–26. doi: 10.1038/nrrheum.2009.209
25. Zaripova LN, Midgley A, Christmas SE, Beresford MW, Baildam EM, Oldershaw RA. Juvenile idiopathic arthritis: from aetiopathogenesis to therapeutic approaches. Pediatr Rheumatol Online J. (2021) 19:135. doi: 10.1186/s12969-021-00629-8
26. Grassi A, Corona F, Casellato A, Carnelli V, Bardare M. Prevalence and outcome of juvenile idiopathic arthritis-associated uveitis and relation to articular disease. J Rheumatol. (2007) 34:1139–45.
27. Carlsson E, Beresford MW, Ramanan AV, Dick AD, Hedrich CM. Juvenile idiopathic arthritis associated uveitis. Children. (2021) 8:646. doi: 10.3390/children8080646
28. Lazăr C, Spîrchez M, Stefan M, Predeteanu D, Nicoară S, Crişan M, et al. Diagnosis and treatment of uveitis associated with juvenile idiopathic arthritis. Med Pharm Rep. (2021) 94:S28–32. doi: 10.15386/mpr-2224
29. Mellins ED, Macaubas C, Grom AA. Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more questions. Nat Rev Rheumatol. (2011) 7:416–26. doi: 10.1038/nrrheum.2011.68
30. Martini A. Systemic juvenile idiopathic arthritis. Autoimmun Rev. (2012) 12:56–9. doi: 10.1016/j.autrev.2012.07.022
31. Minoia F, Davì S, Horne A, Demirkaya E, Bovis F, Li C, et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol. (2014) 66:3160–9. doi: 10.1002/art.38802
32. Boom V, Anton J, Lahdenne P, Quartier P, Ravelli A, Wulffraat NM, et al. Evidence-based diagnosis and treatment of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Pediatr Rheumatol Online J. (2015) 13:55. doi: 10.1186/s12969-015-0055-3
33. Ravelli A, Minoia F, Davì S, Horne A, Bovis F, Pistorio A, et al. 2016 classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Ann Rheum Dis. (2016) 75:481–9. doi: 10.1136/annrheumdis-2015-208982
34. Omoyinmi E, Hamaoui R, Pesenacker A, Nistala K, Moncrieffe H, Ursu S, et al. Th1 and Th17 cell subpopulations are enriched in the peripheral blood of patients with systemic juvenile idiopathic arthritis. Rheumatology. (2012) 51:1881–6. doi: 10.1093/rheumatology/kes162
35. Ombrello MJ, Remmers EF, Tachmazidou I, Grom A, Foell D, Haas J-P, et al. HLA-DRB1*11 and variants of the MHC class II locus are strong risk factors for systemic juvenile idiopathic arthritis. Proc Natl Acad Sci USA. (2015) 112:15970–5. doi: 10.1073/pnas.1520779112
36. Kessel C, Lippitz K, Weinhage T, Hinze C, Wittkowski H, Holzinger D, et al. Proinflammatory cytokine environments can drive interleukin-17 overexpression by γ/δ T cells in systemic juvenile idiopathic arthritis. Arthritis Rheumatol. (2017) 69:1480–94. doi: 10.1002/art.40099
37. Henderson LA, Hoyt KJ, Lee PY, Rao DA, Jonsson AH, Nguyen JP, et al. Th17 reprogramming of T cells in systemic juvenile idiopathic arthritis. JCI Insight. (2020) 5:132508. doi: 10.1172/jci.insight.132508
38. Kessel C, Hedrich CM, Foell D. Innately adaptive or truly autoimmune: is there something unique about systemic juvenile idiopathic arthritis? Arthritis Rheumatol. (2020) 72:210–9. doi: 10.1002/art.41107
39. Stoll ML, Punaro M. Psoriatic juvenile idiopathic arthritis: a tale of two subgroups. Curr Opin Rheumatol. (2011) 23:437–43. doi: 10.1097/BOR.0b013e328348b278
40. Stoll ML, Mellins ED. Psoriatic arthritis in childhood: a commentary on the controversy. Clin Immunol. (2020) 214:108396. doi: 10.1016/j.clim.2020.108396
41. Giancane G, Consolaro A, Lanni S, Davì S, Schiappapietra B, Ravelli A. Juvenile idiopathic arthritis: diagnosis and treatment. Rheumatol Ther. (2016) 3:187–207. doi: 10.1007/s40744-016-0040-4
42. Martini A. It is time to rethink juvenile idiopathic arthritis classification and nomenclature. Ann Rheum Dis. (2012) 71:1437–9. doi: 10.1136/annrheumdis-2012-201388
43. Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R, et al. Toward new classification criteria for juvenile idiopathic arthritis: first steps, pediatric rheumatology international trials organization international consensus. J Rheumatol. (2019) 46:190–7. doi: 10.3899/jrheum.180168
44. Carlens C, Jacobsson L, Brandt L, Cnattingius S, Stephansson O, Askling J. Perinatal characteristics, early life infections and later risk of rheumatoid arthritis and juvenile idiopathic arthritis. Ann Rheum Dis. (2009) 68:1159–64. doi: 10.1136/ard.2008.089342
45. Kindgren E, Ludvigsson J. Infections and antibiotics during fetal life and childhood and their relationship to juvenile idiopathic arthritis: a prospective cohort study. Pediatr Rheumatol Online J. (2021) 19:145. doi: 10.1186/s12969-021-00611-4
46. Rigante D, Bosco A, Esposito S. The etiology of juvenile idiopathic arthritis. Clin Rev Allergy Immunol. (2015) 49:253–61. doi: 10.1007/s12016-014-8460-9
47. Verwoerd A, Ter Haar NM, de Roock S, Vastert SJ, Bogaert D. The human microbiome and juvenile idiopathic arthritis. Pediatr Rheumatol Online J. (2016) 14:55. doi: 10.1186/s12969-016-0114-4
48. Stoll ML, Kumar R, Lefkowitz EJ, Cron RQ, Morrow CD, Barnes S. Fecal metabolomics in pediatric spondyloarthritis implicate decreased metabolic diversity and altered tryptophan metabolism as pathogenic factors. Genes Immun. (2016) 17:400–5. doi: 10.1038/gene.2016.38
49. Xin L, He F, Li S, Zhou Z-X, Ma X-L. Intestinal microbiota and juvenile idiopathic arthritis: current understanding and future prospective. World J Pediatr. (2021) 17:40–51. doi: 10.1007/s12519-020-00371-3
50. Jaakkola JJK, Gissler M. Maternal smoking in pregnancy as a determinant of rheumatoid arthritis and other inflammatory polyarthropathies during the first 7 years of life. Int J Epidemiol. (2005) 34:664–71. doi: 10.1093/ije/dyi006
51. Vicario JL, Martinez-Laso J, Gomez-Reino JJ, Gomez-Reino FJ, Regueiro JR, Corell A, et al. Both HLA class II and class III DNA polymorphisms are linked to juvenile rheumatoid arthritis susceptibility. Clin Immunol Immunopathol. (1990) 56:22–8. doi: 10.1016/0090-1229(90)90165-M
52. Hollenbach JA, Thompson SD, Bugawan TL, Ryan M, Sudman M, Marion M, et al. Juvenile idiopathic arthritis and HLA class I and class II interactions and age-at-onset effects. Arthritis Rheum. (2010) 62:1781–91. doi: 10.1002/art.27424
53. Chistiakov DA, Savost'anov KV, Baranov AA. Genetic background of juvenile idiopathic arthritis. Autoimmunity. (2014) 47:351–60. doi: 10.3109/08916934.2014.889119
54. Hersh AO, Prahalad S. Immunogenetics of juvenile idiopathic arthritis: a comprehensive review. J Autoimmun. (2015) 64:113–24. doi: 10.1016/j.jaut.2015.08.002
55. De Silvestri A, Capittini C, Poddighe D, Marseglia GL, Mascaretti L, Bevilacqua E, et al. HLA-DRB1 alleles and juvenile idiopathic arthritis: diagnostic clues emerging from a meta-analysis. Autoimmun Rev. (2017) 16:1230–6. doi: 10.1016/j.autrev.2017.10.007
56. Hersh AO, Prahalad S. Genetics of juvenile idiopathic arthritis. Rheum Dis Clin North Am. (2017) 43:435–48. doi: 10.1016/j.rdc.2017.04.007
57. Mistry RR, Patro P, Agarwal V, Misra DP. Enthesitis-related arthritis: current perspectives. Open Access Rheumatol. (2019) 11:19–31. doi: 10.2147/OARRR.S163677
58. Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet. (2011) 377:2138–49. doi: 10.1016/S0140-6736(11)60244-4
59. Sikora KA, Grom AA. Update on the pathogenesis and treatment of systemic idiopathic arthritis. Curr Opin Pediatr. (2011) 23:640–6. doi: 10.1097/MOP.0b013e32834cba24
60. Lin Y-T, Wang C-T, Gershwin ME, Chiang B-L. The pathogenesis of oligoarticular/polyarticular vs systemic juvenile idiopathic arthritis. Autoimmun Rev. (2011) 10:482–9. doi: 10.1016/j.autrev.2011.02.001
61. Schmidt T, Berthold E, Arve-Butler S, Gullstrand B, Mossberg A, Kahn F, et al. Children with oligoarticular juvenile idiopathic arthritis have skewed synovial monocyte polarization pattern with functional impairment-a distinct inflammatory pattern for oligoarticular juvenile arthritis. Arthritis Res Ther. (2020) 22:186. doi: 10.1186/s13075-020-02279-9
62. Arve-Butler S, Schmidt T, Mossberg A, Berthold E, Gullstrand B, Bengtsson AA, et al. Synovial fluid neutrophils in oligoarticular juvenile idiopathic arthritis have an altered phenotype and impaired effector functions. Arthritis Res Ther. (2021) 23:109. doi: 10.1186/s13075-021-02483-1
63. Metzemaekers M, Malengier-Devlies B, Yu K, Vandendriessche S, Yserbyt J, Matthys P, et al. Synovial fluid neutrophils from patients with juvenile idiopathic arthritis display a hyperactivated phenotype. Arthritis Rheumatol. (2021) 73:875–84. doi: 10.1002/art.41605
64. Murray KJ, Luyrink L, Grom AA, Passo MH, Emery H, Witte D, et al. Immunohistological characteristics of T cell infiltrates in different forms of childhood onset chronic arthritis. J Rheumatol. (1996) 23:2116–24.
65. Gattorno M, Prigione I, Morandi F, Gregorio A, Chiesa S, Ferlito F, et al. Phenotypic and functional characterisation of CCR7+ and CCR7- CD4+ memory T cells homing to the joints in juvenile idiopathic arthritis. Arthritis Res Ther. (2005) 7:R256–67. doi: 10.1186/ar1485
66. Agarwal S, Misra R, Aggarwal A. Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. J Rheumatol. (2008) 35:515–9.
67. Nistala K, Moncrieffe H, Newton KR, Varsani H, Hunter P, Wedderburn LR. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. (2008) 58:875–87. doi: 10.1002/art.23291
68. Olivito B, Simonini G, Ciullini S, Moriondo M, Betti L, Gambineri E, et al. Th17 transcription factor RORC2 is inversely correlated with FOXP3 expression in the joints of children with juvenile idiopathic arthritis. J Rheumatol. (2009) 36:2017–24. doi: 10.3899/jrheum.090066
69. Finnegan S, Clarke S, Gibson D, McAllister C, Rooney M. Synovial membrane immunohistology in early untreated juvenile idiopathic arthritis: differences between clinical subgroups. Ann Rheum Dis. (2011) 70:1842–50. doi: 10.1136/ard.2010.148635
70. Amariglio N, Klein A, Dagan L, Lev A, Padeh S, Rechavi G, et al. T-cell compartment in synovial fluid of pediatric patients with JIA correlates with disease phenotype. J Clin Immunol. (2011) 31:1021–8. doi: 10.1007/s10875-011-9580-0
71. Cosmi L, Cimaz R, Maggi L, Santarlasci V, Capone M, Borriello F, et al. Evidence of the transient nature of the Th17 phenotype of CD4+CD161+ T cells in the synovial fluid of patients with juvenile idiopathic arthritis. Arthritis Rheum. (2011) 63:2504–15. doi: 10.1002/art.30332
72. Haufe S, Haug M, Schepp C, Kuemmerle-Deschner J, Hansmann S, Rieber N, et al. Impaired suppression of synovial fluid CD4+CD25- T cells from patients with juvenile idiopathic arthritis by CD4+CD25+ Treg cells. Arthritis Rheum. (2011) 63:3153–62. doi: 10.1002/art.30503
73. Berkun Y, Bendersky A, Gerstein M, Goldstein I, Padeh S, Bank I. GammadeltaT cells in juvenile idiopathic arthritis: higher percentages of synovial Vdelta1+ and Vgamma9+ T cell subsets are associated with milder disease. J Rheumatol. (2011) 38:1123–9. doi: 10.3899/jrheum.100938
74. Bendersky A, Marcu-Malina V, Berkun Y, Gerstein M, Nagar M, Goldstein I, et al. Cellular interactions of synovial fluid γδ T cells in juvenile idiopathic arthritis. J Immunol. (2012) 188:4349–59. doi: 10.4049/jimmunol.1102403
75. Stelmaszczyk-Emmel A, Jackowska T, Rutkowska-Sak L, Marusak-Banacka M, Wasik M. Identification, frequency, activation and function of CD4+ CD25(high)FoxP3+ regulatory T cells in children with juvenile idiopathic arthritis. Rheumatol Int. (2012) 32:1147–54. doi: 10.1007/s00296-010-1728-3
76. Szymańska-Kałuza J, Cebula-Obrzut B, Smolewski P, Stanczyk J, Smolewska E. Imbalance of Th17 and T-regulatory cells in peripheral blood and synovial fluid in treatment naïve children with juvenile idiopathic arthritis. Cent Eur J Immunol. (2014) 39:71–6. doi: 10.5114/ceji.2014.42128
77. Oberle EJ, Harris JG, Verbsky JW. Polyarticular juvenile idiopathic arthritis - epidemiology and management approaches. Clin Epidemiol. (2014) 6:379–93. doi: 10.2147/CLEP.S53168
78. Wu S-A, Yeh K-W, Lee W-I, Yao T-C, Huang J-L. Persistent improper upregulation of Th17 and TReg cells in patients with juvenile idiopathic arthritis. J Microbiol Immunol Infect. (2016) 49:402–8. doi: 10.1016/j.jmii.2014.07.002
79. Spreafico R, Rossetti M, van Loosdregt J, Wallace CA, Massa M, Magni-Manzoni S, et al. circulating reservoir of pathogenic-like CD4+ T cells shares a genetic and phenotypic signature with the inflamed synovial micro-environment. Ann Rheum Dis. (2016) 75:459–65. doi: 10.1136/annrheumdis-2014-206226
80. Rosser EC, Lom H, Bending D, Duurland CL, Bajaj-Elliott M, Wedderburn LR. Innate lymphoid cells and T cells contribute to the interleukin-17A signature detected in the synovial fluid of patients with juvenile idiopathic arthritis. Arthritis Rheumatol. (2019) 71:460–7. doi: 10.1002/art.40731
81. Mahendra A, Misra R, Aggarwal A. Th1 and Th17 predominance in the enthesitis-related arthritis form of juvenile idiopathic arthritis. J Rheumatol. (2009) 36:1730–6. doi: 10.3899/jrheum.081179
82. Colbert RA. Classification of juvenile spondyloarthritis: enthesitis-related arthritis and beyond. Nat Rev Rheumatol. (2010) 6:477–85. doi: 10.1038/nrrheum.2010.103
83. Sherlock JP, Joyce-Shaikh B, Turner SP, Chao C-C, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. (2012) 18:1069–76. doi: 10.1038/nm.2817
84. Jacques P, Lambrecht S, Verheugen E, Pauwels E, Kollias G, Armaka M, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis. (2014) 73:437–45. doi: 10.1136/annrheumdis-2013-203643
85. Gmuca S, Weiss PF. Juvenile spondyloarthritis. Curr Opin Rheumatol. (2015) 27:364–72. doi: 10.1097/BOR.0000000000000185
86. Fisher C, Ciurtin C, Leandro M, Sen D, Wedderburn LR. Similarities and differences between juvenile and adult spondyloarthropathies. Front Med. (2021) 8:681621. doi: 10.3389/fmed.2021.681621
87. Correll CK, Binstadt BA. Advances in the pathogenesis and treatment of systemic juvenile idiopathic arthritis. Pediatr Res. (2014) 75:176–83. doi: 10.1038/pr.2013.187
88. de Benedetti F, Massa M, Robbioni P, Ravelli A, Burgio GR, Martini A. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. (1991) 34:1158–63. doi: 10.1002/art.1780340912
89. Yilmaz M, Kendirli SG, Altintas D, Bingöl G, Antmen B. Cytokine levels in serum of patients with juvenile rheumatoid arthritis. Clin Rheumatol. (2001) 20:30–5. doi: 10.1007/s100670170100
90. Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. (2005) 201:1479–86. doi: 10.1084/jem.20050473
91. Fall N, Barnes M, Thornton S, Luyrink L, Olson J, Ilowite NT, et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. (2007) 56:3793–804. doi: 10.1002/art.22981
92. Macaubas C, Nguyen K, Deshpande C, Phillips C, Peck A, Lee T, et al. Distribution of circulating cells in systemic juvenile idiopathic arthritis across disease activity states. Clin Immunol. (2010) 134:206–16. doi: 10.1016/j.clim.2009.09.010
93. Hinze CH, Fall N, Thornton S, Mo JQ, Aronow BJ, Layh-Schmitt G, et al. Immature cell populations and an erythropoiesis gene-expression signature in systemic juvenile idiopathic arthritis: implications for pathogenesis. Arthritis Res Ther. (2010) 12:R123. doi: 10.1186/ar3061
94. Brown RA, Henderlight M, Do T, Yasin S, Grom AA, DeLay M, et al. Neutrophils from children with systemic juvenile idiopathic arthritis exhibit persistent proinflammatory activation despite long-standing clinically inactive disease. Front Immunol. (2018) 9:2995. doi: 10.3389/fimmu.2018.02995
95. Vilaiyuk S, Lerkvaleekul B, Soponkanaporn S, Setthaudom C, Buranapraditkun S. Correlations between serum interleukin 6, serum soluble interleukin 6 receptor, and disease activity in systemic juvenile idiopathic arthritis patients treated with or without tocilizumab. Cent Eur J Immunol. (2019) 44:150–8. doi: 10.5114/ceji.2019.87066
96. Holzinger D, Tenbrock K, Roth J. Alarmins of the S100-family in juvenile autoimmune and auto-inflammatory diseases. Front Immunol. (2019) 10:182. doi: 10.3389/fimmu.2019.00182
97. Yasin S, Fall N, Brown RA, Henderlight M, Canna SW, Girard-Guyonvarc'h C, et al. IL-18 as a biomarker linking systemic juvenile idiopathic arthritis and macrophage activation syndrome. Rheumatology. (2020) 59:361–6. doi: 10.1093/rheumatology/kez282
98. Aljaberi N, Tronconi E, Schulert G, Grom AA, Lovell DJ, Huggins JL, et al. The use of S100 proteins testing in juvenile idiopathic arthritis and autoinflammatory diseases in a pediatric clinical setting: a retrospective analysis. Pediatr Rheumatol Online J. (2020) 18:7. doi: 10.1186/s12969-020-0398-2
99. Spelling P, Bonfá E, Caparbo VF, Pereira RMR. Osteoprotegerin/RANKL system imbalance in active polyarticular-onset juvenile idiopathic arthritis: a bone damage biomarker? Scand J Rheumatol. (2008) 37:439–44. doi: 10.1080/03009740802116224
100. Pradsgaard DØ, Spannow AH, Heuck C, Herlin T. Decreased cartilage thickness in juvenile idiopathic arthritis assessed by ultrasonography. J Rheumatol. (2013) 40:1596–603. doi: 10.3899/jrheum.121077
101. Swidrowska J, Smolewski P, Stańczyk J, Smolewska E. Serum angiogenesis markers and their correlation with ultrasound-detected synovitis in juvenile idiopathic arthritis. J Immunol Res. (2015) 2015:741457. doi: 10.1155/2015/741457
102. Ventura-Ríos L Faugier E Barzola L De la Cruz-Becerra LB Sánchez-Bringas G García AR Reliability Reliability of ultrasonography to detect inflammatory lesions and structural damage in juvenile idiopathic arthritis. Pediatr Rheumatol Online J. (2018) 16:58. doi: 10.1186/s12969-018-0275-4
103. Swidrowska-Jaros J, Smolewska E. A fresh look at angiogenesis in juvenile idiopathic arthritis. Cent Eur J Immunol. (2018) 43:325–30. doi: 10.5114/ceji.2018.80052
104. Mitra S, Samui PP, Samanta M, Mondal RK, Hazra A, Mandal K, et al. Ultrasound detected changes in joint cartilage thickness in juvenile idiopathic arthritis. Int J Rheum Dis. (2019) 22:1263–70. doi: 10.1111/1756-185X.13584
105. Michalski E, Ostrowska M, Gietka P, Sudoł-Szopińska I. Magnetic resonance imaging of the knee joint in juvenile idiopathic arthritis. Reumatologia. (2020) 58:416–23. doi: 10.5114/reum.2020.102007
106. Struglics A, Saleh R, Sundberg E, Olsson M, Erlandsson Harris H, Aulin C. Juvenile idiopathic arthritis patients have a distinct cartilage and bone biomarker profile that differs from healthy and knee-injured children. Clin Exp Rheumatol. (2020) 38:355–65.
107. Wojdas M, Dabkowska K, Winsz-Szczotka K. Alterations of extracellular matrix components in the course of juvenile idiopathic arthritis. Metabolites. (2021) 11:132. doi: 10.3390/metabo11030132
108. Szer W, Sierakowska H, Szer IS. Antinuclear antibody profile in juvenile rheumatoid arthritis. J Rheumatol. (1991) 18:401–8.
109. van Rossum M, van Soesbergen R, de Kort S, ten Cate R, Zwinderman AH, de Jong B, et al. Anti-cyclic citrullinated peptide (anti-CCP) antibodies in children with juvenile idiopathic arthritis. J Rheumatol. (2003) 30:825–8.
110. Ravelli A, Felici E, Magni-Manzoni S, Pistorio A, Novarini C, Bozzola E, et al. Patients with antinuclear antibody-positive juvenile idiopathic arthritis constitute a homogeneous subgroup irrespective of the course of joint disease. Arthritis Rheum. (2005) 52:826–32. doi: 10.1002/art.20945
111. Syed RH, Gilliam BE, Moore TL. Rheumatoid factors and anticyclic citrullinated peptide antibodies in pediatric rheumatology. Curr Rheumatol Rep. (2008) 10:156–63. doi: 10.1007/s11926-008-0027-4
112. Omar A, Abo-Elyoun I, Hussein H, Nabih M, Atwa H, Gad S, et al. Anti-cyclic citrullinated peptide (anti-CCP) antibody in juvenile idiopathic arthritis (JIA): correlations with disease activity and severity of joint damage (a multicenter trial). Joint Bone Spine. (2013) 80:38–43. doi: 10.1016/j.jbspin.2012.03.008
113. Berntson L, Nordal E, Fasth A, Aalto K, Herlin T, Nielsen S, et al. Anti-type II collagen antibodies, anti-CCP, IgA RF and IgM RF are associated with joint damage, assessed eight years after onset of juvenile idiopathic arthritis (JIA). Pediatr Rheumatol Online J. (2014) 12:22. doi: 10.1186/1546-0096-12-22
114. Hamooda M, Fouad H, Galal N, Sewelam N, Megahed D. Anti-cyclic citrullinated peptide antibodies in children with juvenile idiopathic arthritis. Electron Physician. (2016) 8:2897–903. doi: 10.19082/2897
115. Sur LM, Floca E, Sur DG, Colceriu MC, Samasca G, Sur G. Antinuclear antibodies: marker of diagnosis and evolution in autoimmune diseases. Lab Med. (2018) 49:e62–73. doi: 10.1093/labmed/lmy024
116. Altintas A, Ozel A, Okur N, Okur N, Cil T, Pasa S, et al. Prevalence and clinical significance of elevated antinuclear antibody test in children and adult patients with idiopathic thrombocytopenic purpura. J Thromb Thrombolysis. (2007) 24:163–8. doi: 10.1007/s11239-007-0031-y
117. Barahona-Garrido J, Camacho-Escobedo J, García-Martínez CI, Tocay H, Cabiedes J, Yamamoto-Furusho JK. Antinuclear antibodies: a marker associated with steroid dependence in patients with ulcerative colitis. Inflamm Bowel Dis. (2009) 15:1039–43. doi: 10.1002/ibd.20852
118. Radic M, Herrmann M, van der Vlag J, Rekvig OP. Regulatory and pathogenetic mechanisms of autoantibodies in SLE. Autoimmunity. (2011) 44:349–56. doi: 10.3109/08916934.2010.536794
119. Campanilho-Marques R, Bogas M, Ramos F, Santos MJ, Fonseca JE. Prognostic value of antinuclear antibodies in juvenile idiopathic arthritis and anterior uveitis. Results from a systematic literature review. Acta Reumatol Port. (2014) 39:116–22.
120. Segni M, Pucarelli I, Truglia S, Turriziani I, Serafinelli C, Conti F. High prevalence of antinuclear antibodies in children with thyroid autoimmunity. J Immunol Res. (2014) 2014:150239. doi: 10.1155/2014/150239
121. Maślińska M, Mańczak M, Wojciechowska B, Kwiatkowska B. The prevalence of ANA antibodies, anticentromere antibodies, and anti-cyclic citrullinated peptide antibodies in patients with primary Sjögren's syndrome compared to patients with dryness symptoms without primary Sjögren's syndrome confirmation. Reumatologia. (2017) 55:113–9. doi: 10.5114/reum.2017.68909
122. Ahsan T, Erum U, Dahani A, Khowaja D. Clinical and immunological profile in patients with mixed connective tissue disease. J Pak Med Assoc. (2018) 68:959–62.
123. Cha HJ, Hwang J, Lee LE, Park Y, Song JJ. The significance of cytoplasmic antinuclear antibody patterns in autoimmune liver disease. PLoS ONE. (2021) 16:e0244950. doi: 10.1371/journal.pone.0244950
124. Paknikar SS, Crowson CS, Davis JM, Thanarajasingam U. Exploring the role of antinuclear antibody positivity in the diagnosis, treatment, and health outcomes of patients with rheumatoid arthritis. ACR Open Rheumatol. (2021) 3:422–6. doi: 10.1002/acr2.11271
125. Sharma A, Bhattarai D, Gupta A, Guleria S, Rawat A, Vignesh P, et al. Autoantibody profile of children with juvenile dermatomyositis. Indian J Pediatr. (2021) 88:1170–3. doi: 10.1007/s12098-021-03680-1
126. Glerup M, Herlin T, Twilt M. Remission rate is not dependent on the presence of antinuclear antibodies in juvenile idiopathic arthritis. Clin Rheumatol. (2017) 36:671–6. doi: 10.1007/s10067-017-3540-x
127. Guillaume S, Prieur AM, Coste J, Job-Deslandre C. Long-term outcome and prognosis in oligoarticular-onset juvenile idiopathic arthritis. Arthritis Rheum. (2000) 43:1858–65. doi: 10.1002/1529-0131(200008)43:8<1858::AID-ANR23>3.0.CO;2-A
128. Saurenmann RK, Levin AV, Feldman BM, Laxer RM, Schneider R, Silverman ED. Risk factors for development of uveitis differ between girls and boys with juvenile idiopathic arthritis. Arthritis Rheum. (2010) 62:1824–8. doi: 10.1002/art.27416
129. Rose HM, Ragan C. Differential agglutination of normal and sensitized sheep erythrocytes by sera of patients with rheumatoid arthritis. Proc Soc Exp Biol Med. (1948) 68:1–6. doi: 10.3181/00379727-68-16375
130. Waaler E. On the occurrence of a factor in human serum activating the specific agglutintion of sheep blood corpuscles. APMIS. (2007) 115:422–38. doi: 10.1111/j.1600-0463.2007.apm_682a.x
131. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. (2010) 62:2569–81. doi: 10.1002/art.27584
132. Soltys AI, Axford JS, Sutton BJ. Rheumatoid factors: where are we now? Ann Rheum Dis. (1997) 56:285–6. doi: 10.1136/ard.56.5.285
133. Ingegnoli F, Castelli R, Gualtierotti R. Rheumatoid factors: clinical applications. Dis Markers. (2013) 35:727–34. doi: 10.1155/2013/726598
134. Toumbis A, Franklin EC, McEWEN C, Kuttner AG. Clinical and serologic observations in patients with juvenile rheumatoid arthritis and their relatives. J Pediatr. (1963) 62:463–73. doi: 10.1016/S0022-3476(63)80001-3
135. Eichenfield AH, Athreya BH, Doughty RA, Cebul RD. Utility of rheumatoid factor in the diagnosis of juvenile rheumatoid arthritis. Pediatrics. (1986) 78:480–4. doi: 10.1542/peds.78.3.480
136. Fantini F, Gerloni V, Gattinara M, Cimaz R, Arnoldi C, Lupi E. Remission in juvenile chronic arthritis: a cohort study of 683 consecutive cases with a mean 10 year followup. J Rheumatol. (2003) 30:579–84.
137. Flatø B, Lien G, Smerdel A, Vinje O, Dale K, Johnston V, et al. Prognostic factors in juvenile rheumatoid arthritis: a case-control study revealing early predictors and outcome after 149 years. J Rheumatol. (2003) 30:386–93.
138. Gilliam BE, Chauhan AK, Low JM, Moore TL. Measurement of biomarkers in juvenile idiopathic arthritis patients and their significant association with disease severity: a comparative study. Clin Exp Rheumatol. (2008) 26:492–7.
139. Hinks A, Marion MC, Cobb J, Comeau ME, Sudman M, Ainsworth HC, et al. Brief report: the genetic profile of rheumatoid factor-positive polyarticular juvenile idiopathic arthritis resembles that of adult rheumatoid arthritis. Arthritis Rheumatol. (2018) 70:957–62. doi: 10.1002/art.40443
140. Onuora S. Genetics: subtype of JIA is genetically similar to adult RA. Nat Rev Rheumatol. (2018) 14:181. doi: 10.1038/nrrheum.2018.30
141. Carson DA, Chen PP, Kipps TJ. New roles for rheumatoid factor. J Clin Invest. (1991) 87:379–83. doi: 10.1172/JCI115007
142. Børretzen M, Chapman C, Natvig JB, Thompson KM. Differences in mutational patterns between rheumatoid factors in health and disease are related to variable heavy chain family and germ-line gene usage. Eur J Immunol. (1997) 27:735–41. doi: 10.1002/eji.1830270323
143. Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. (2002) 416:603–7. doi: 10.1038/416603a
144. Derksen VFaM, Huizinga TWJ, van der Woude D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin Immunopathol. (2017) 39:437–46. doi: 10.1007/s00281-017-0627-z
145. van Delft MAM, Huizinga TWJ. An overview of autoantibodies in rheumatoid arthritis. J Autoimmun. (2020) 110:102392. doi: 10.1016/j.jaut.2019.102392
146. Brunner J, Sitzmann FC. The diagnostic value of anti-cyclic citrullinated peptide (CCP) antibodies in children with Juvenile Idiopathic Arthritis. Clin Exp Rheumatol. (2006) 24:449–51.
147. Wang Y, Pei F, Wang X, Sun Z, Hu C, Dou H. Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody for juvenile idiopathic arthritis. J Immunol Res. (2015) 2015:915276. doi: 10.1155/2015/915276
148. Peckham H, Cambridge G, Bourke L, Sen D, Radziszewska A, Leandro M, et al. Antibodies to cyclic citrullinated peptides in patients with juvenile idiopathic arthritis and patients with rheumatoid arthritis: shared expression of the inherently autoreactive 9G4 idiotype. Arthritis Rheumatol. (2017) 69:1387–95. doi: 10.1002/art.40117
149. Cambridge G, Moura RA, Santos T, Khawaja AA, Polido-Pereira J, Canhão H, et al. Expression of the inherently autoreactive idiotope 9G4 on autoantibodies to citrullinated peptides and on rheumatoid factors in patients with early and established rheumatoid arthritis. PLoS ONE. (2014) 9:e107513. doi: 10.1371/journal.pone.0107513
150. Spârchez M, Miu N, Bolba C, Iancu M, Spârchez Z, Rednic S. Evaluation of anti-cyclic citrullinated peptide antibodies may be beneficial in RF-negative juvenile idiopathic arthritis patients. Clin Rheumatol. (2016) 35:601–7. doi: 10.1007/s10067-015-2971-5
151. Selvaag AM, Kirkhus E, Törnqvist L, Lilleby V, Aulie HA, Flatø B. Radiographic damage in hands and wrists of patients with juvenile idiopathic arthritis after 29 years of disease duration. Pediatr Rheumatol Online J. (2017) 15:20. doi: 10.1186/s12969-017-0151-7
152. Valesini G, Gerardi MC, Iannuccelli C, Pacucci VA, Pendolino M, Shoenfeld Y. Citrullination and autoimmunity. Autoimmun Rev. (2015) 14:490–7. doi: 10.1016/j.autrev.2015.01.013
153. Alghamdi M, Alasmari D, Assiri A, Mattar E, Aljaddawi AA, Alattas SG, et al. An overview of the intrinsic role of citrullination in autoimmune disorders. J Immunol Res. (2019) 2019:7592851. doi: 10.1155/2019/7592851
154. Gilliam BE, Reed MR, Chauhan AK, Dehlendorf AB, Moore TL. Significance of complement components C1q and C4 bound to circulating immune complexes in juvenile idiopathic arthritis: support for classical complement pathway activation. Clin Exp Rheumatol. (2011) 29:1049–56.
155. Moore TL. Immune complexes in juvenile idiopathic arthritis. Front Immunol. (2016) 7:177. doi: 10.3389/fimmu.2016.00177
156. Souto-Carneiro MM, Mahadevan V, Takada K, Fritsch-Stork R, Nanki T, Brown M, et al. Alterations in peripheral blood memory B cells in patients with active rheumatoid arthritis are dependent on the action of tumour necrosis factor. Arthritis Res Ther. (2009) 11:R84. doi: 10.1186/ar2718
157. Moura RA, Weinmann P, Pereira PA, Caetano-Lopes J, Canhão H, Sousa E, et al. Alterations on peripheral blood B-cell subpopulations in very early arthritis patients. Rheumatology. (2010) 49:1082–92. doi: 10.1093/rheumatology/keq029
158. Cascão R, Moura RA, Perpétuo I, Canhão H, Vieira-Sousa E, Mourão AF, et al. Identification of a cytokine network sustaining neutrophil and Th17 activation in untreated early rheumatoid arthritis. Arthritis Res Ther. (2010) 12:R196. doi: 10.1186/ar3168
159. Moura RA, Cascão R, Perpétuo I, Canhão H, Vieira-Sousa E, Mourão AF, et al. Cytokine pattern in very early rheumatoid arthritis favours B-cell activation and survival. Rheumatology. (2011) 50:278–82. doi: 10.1093/rheumatology/keq338
160. Moura RA, Graca L, Fonseca JE. To B or not to B the conductor of rheumatoid arthritis orchestra. Clin Rev Allergy Immunol. (2012) 43:281–91. doi: 10.1007/s12016-012-8318-y
161. Moura RA, Canhão H, Polido-Pereira J, Rodrigues AM, Navalho M, Mourão AF, et al. BAFF and TACI gene expression are increased in patients with untreated very early rheumatoid arthritis. J Rheumatol. (2013) 40:1293–302. doi: 10.3899/jrheum.121110
162. Moura RA, Quaresma C, Vieira AR, Gonçalves MJ, Polido-Pereira J, Romão VC, et al. B-cell phenotype and IgD-CD27- memory B cells are affected by TNF-inhibitors and tocilizumab treatment in rheumatoid arthritis. PLoS ONE. (2017) 12:e0182927. doi: 10.1371/journal.pone.0182927
163. Amaral-Silva D, Gonçalves R, Torrão RC, Torres R, Falcão S, Gonçalves MJ, et al. Direct tissue-sensing reprograms TLR4+ Tfh-like cells inflammatory profile in the joints of rheumatoid arthritis patients. Commun Biol. (2021) 4:1135. doi: 10.1038/s42003-021-02659-0
164. Zhou S, Lu H, Xiong M. Identifying immune cell infiltration and effective diagnostic biomarkers in rheumatoid arthritis by bioinformatics analysis. Front Immunol. (2021) 12:726747. doi: 10.3389/fimmu.2021.726747
165. Wu X, Liu Y, Jin S, Wang M, Jiao Y, Yang B, et al. Single-cell sequencing of immune cells from anticitrullinated peptide antibody positive and negative rheumatoid arthritis. Nat Commun. (2021) 12:4977. doi: 10.1038/s41467-021-25246-7
166. Tu J, Huang W, Zhang W, Mei J, Zhu C. A tale of two immune cells in rheumatoid arthritis: the crosstalk between macrophages and T cells in the synovium. Front Immunol. (2021) 12:655477. doi: 10.3389/fimmu.2021.655477
167. Yeo L, Toellner K-M, Salmon M, Filer A, Buckley CD, Raza K, et al. Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann Rheum Dis. (2011) 70:2022–8. doi: 10.1136/ard.2011.153312
168. Armas-González E, Díaz-Martín A, Domínguez-Luis MJ, Arce-Franco MT, Herrera-García A, Hernández-Hernández MV, et al. Differential antigen-presenting B cell phenotypes from synovial microenvironment of patients with rheumatoid and psoriatic arthritis. J Rheumatol. (2015) 42:1825–34. doi: 10.3899/jrheum.141577
169. Meednu N, Zhang H, Owen T, Sun W, Wang V, Cistrone C, et al. Production of RANKL by memory B cells: a link between B cells and bone erosion in rheumatoid arthritis. Arthritis Rheumatol. (2016) 68:805–16. doi: 10.1002/art.39489
170. Fischer J, Dirks J, Haase G, Holl-Wieden A, Hofmann C, Girschick H, et al. IL-21+ CD4+ T helper cells co-expressing IFN-γ and TNF-α accumulate in the joints of antinuclear antibody positive patients with juvenile idiopathic arthritis. Clin Immunol. (2020) 217:108484. doi: 10.1016/j.clim.2020.108484
171. Dirks J, Fischer J, Haase G, Holl-Wieden A, Hofmann C, Girschick H, et al. CD21lo/-CD27-IgM- double-negative B cells accumulate in the joints of patients with antinuclear antibody-positive juvenile idiopathic arthritis. Front Pediatr. (2021) 9:635815. doi: 10.3389/fped.2021.635815
172. Fischer J, Dirks J, Klaussner J, Haase G, Holl-Wieden A, Hofmann C, et al. Effect of clonally expanded PD-1high CXCR5-CD4+ peripheral T helper cells on B cell differentiation in the joints of patients with antinuclear antibody-positive juvenile idiopathic arthritis. Arthritis Rheumatol. (2022) 74:150–62. doi: 10.1002/art.41913
173. Kruithof E, Van den Bossche V, De Rycke L, Vandooren B, Joos R, Cañete JD, et al. Distinct synovial immunopathologic characteristics of juvenile-onset spondylarthritis and other forms of juvenile idiopathic arthritis. Arthritis Rheum. (2006) 54:2594–604. doi: 10.1002/art.22024
174. Kalinina Ayuso V, van Dijk MR, de Boer JH. Infiltration of plasma cells in the iris of children with ANA-positive anterior uveitis. Invest Ophthalmol Vis Sci. (2015) 56:6770–8. doi: 10.1167/iovs.15-17351
175. Wildschütz L, Ackermann D, Witten A, Kasper M, Busch M, Glander S, et al. Transcriptomic and proteomic analysis of iris tissue and aqueous humor in juvenile idiopathic arthritis-associated uveitis. J Autoimmun. (2019) 100:75–83. doi: 10.1016/j.jaut.2019.03.004
176. Licciardi F, Ceci M, Toppino C, Turco M, Martino S, Ricotti E, et al. Low synovial double negative T and γδ T cells predict longer free-disease survival in oligoarticular JIA. Cytometry B Clin Cytom. (2018) 94:423–7. doi: 10.1002/cyto.b.21597
177. Marasco E, Aquilani A, Cascioli S, Moneta GM, Caiello I, Farroni C, et al. Switched memory B cells are increased in oligoarticular and polyarticular juvenile idiopathic arthritis and their change over time is related to response to tumor necrosis factor inhibitors. Arthritis Rheumatol. (2018) 70:606–15. doi: 10.1002/art.40410
178. Zhao Q, Jung LK. Frequency of CD19+CD24hiCD38hi regulatory B cells is decreased in peripheral blood and synovial fluid of patients with juvenile idiopathic arthritis: a preliminary study. Pediatr Rheumatol Online J. (2018) 16:44. doi: 10.1186/s12969-018-0262-9
179. Nagy A, Mosdosi B, Simon D, Dergez T, Berki T. Peripheral blood lymphocyte analysis in oligo- and polyarticular juvenile idiopathic arthritis patients receiving methotrexate or adalimumab therapy: a cross-sectional study. Front Pediatr. (2020) 8:614354. doi: 10.3389/fped.2020.614354
180. Vernino LA, Pisetsky DS, Lipsky PE. Analysis of the expression of CD5 by human B cells and correlation with functional activity. Cell Immunol. (1992) 139:185–97. doi: 10.1016/0008-8749(92)90111-2
181. Pers JO, Jamin C, Predine-Hug F, Lydyard P, Youinou P. The role of CD5-expressing B cells in health and disease (review). Int J Mol Med. (1999) 3:239–45. doi: 10.3892/ijmm.3.3.239
182. Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. (2009) 182:4116–26. doi: 10.4049/jimmunol.0803391
183. Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. (2013) 5:173ra23. doi: 10.1126/scitranslmed.3005407
184. Simon Q, Pers J-O, Cornec D, Le Pottier L, Mageed RA, Hillion S. In-depth characterization of CD24(high)CD38(high) transitional human B cells reveals different regulatory profiles. J Allergy Clin Immunol. (2016) 137:1577–84.e10. doi: 10.1016/j.jaci.2015.09.014
185. Nova-Lamperti E, Fanelli G, Becker PD, Chana P, Elgueta R, Dodd PC, et al. IL-10-produced by human transitional B-cells down-regulates CD86 expression on B-cells leading to inhibition of CD4+T-cell responses. Sci Rep. (2016) 6:20044. doi: 10.1038/srep20044
186. Hasan MM, Thompson-Snipes L, Klintmalm G, Demetris AJ, O'Leary J, Oh S, et al. CD24hiCD38hi and CD24hiCD27+ human regulatory B cells display common and distinct functional characteristics. J Immunol. (2019) 203:2110–20. doi: 10.4049/jimmunol.1900488
187. Taher TE, Ong VH, Bystrom J, Hillion S, Simon Q, Denton CP, et al. Association of defective regulation of autoreactive interleukin-6-producing transitional B lymphocytes with disease in patients with systemic sclerosis. Arthritis Rheumatol. (2018) 70:450–61. doi: 10.1002/art.40390
188. Piper CJM, Wilkinson MGL, Deakin CT, Otto GW, Dowle S, Duurland CL, et al. CD19+CD24hiCD38hi B cells are expanded in juvenile dermatomyositis and exhibit a pro-inflammatory phenotype after activation through toll-like receptor 7 and interferon-α. Front Immunol. (2018) 9:1372. doi: 10.3389/fimmu.2018.01372
189. Liu M, Guo Q, Wu C, Sterlin D, Goswami S, Zhang Y, et al. Type I interferons promote the survival and proinflammatory properties of transitional B cells in systemic lupus erythematosus patients. Cell Mol Immunol. (2019) 16:367–79. doi: 10.1038/s41423-018-0010-6
190. Kalampokis I, Venturi GM, Poe JC, Dvergsten JA, Sleasman JW, Tedder TF. The regulatory B cell compartment expands transiently during childhood and is contracted in children with autoimmunity. Arthritis Rheumatol. (2017) 69:225–38. doi: 10.1002/art.39820
191. Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. (2010) 32:129–40. doi: 10.1016/j.immuni.2009.11.009
192. Aybar LT, McGregor JG, Hogan SL, Hu Y, Mendoza CE, Brant EJ, et al. Reduced CD5(+) CD24(hi) CD38(hi) and interleukin-10(+) regulatory B cells in active anti-neutrophil cytoplasmic autoantibody-associated vasculitis permit increased circulating autoantibodies. Clin Exp Immunol. (2015) 180:178–88. doi: 10.1111/cei.12483
193. Wang X, Zhu Y, Zhang M, Wang H, Jiang Y, Gao P. Ulcerative colitis is characterized by a decrease in regulatory B cells. J Crohns Colitis. (2016) 10:1212–23. doi: 10.1093/ecco-jcc/jjw074
194. Heinemann K, Wilde B, Hoerning A, Tebbe B, Kribben A, Witzke O, et al. Decreased IL-10(+) regulatory B cells (Bregs) in lupus nephritis patients. Scand J Rheumatol. (2016) 45:312–6. doi: 10.3109/03009742.2015.1126346
195. Chen Q, Lai L, Chi X, Lu X, Wu H, Sun J, et al. CD19+CD24hiCD38hi B cell dysfunction in primary biliary cholangitis. Mediators Inflamm. (2020) 2020:3019378. doi: 10.1155/2020/3019378
196. Madson KL, Moore TL, Lawrence JM, Osborn TG. Cytokine levels in serum and synovial fluid of patients with juvenile rheumatoid arthritis. J Rheumatol. (1994) 21:2359–63.
197. de Jager W, Hoppenreijs EPAH, Wulffraat NM, Wedderburn LR, Kuis W, Prakken BJ. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis. (2007) 66:589–98. doi: 10.1136/ard.2006.061853
198. van den Ham H-J, de Jager W, Bijlsma JWJ, Prakken BJ, de Boer RJ. Differential cytokine profiles in juvenile idiopathic arthritis subtypes revealed by cluster analysis. Rheumatology. (2009) 48:899–905. doi: 10.1093/rheumatology/kep125
199. Gheita TA, Bassyouni IH, Emad Y, el-Din AMN, Abdel-Rasheed E, Hussein H. Elevated BAFF (BLyS) and APRIL in Juvenile idiopathic arthritis patients: relation to clinical manifestations and disease activity. Joint Bone Spine. (2012) 79:285–90. doi: 10.1016/j.jbspin.2011.05.020
200. Walters HM, Pan N, Lehman TJA, Adams A, Kalliolias GD, Zhu YS, et al. The impact of disease activity and tumour necrosis factor-α inhibitor therapy on cytokine levels in juvenile idiopathic arthritis. Clin Exp Immunol. (2016) 184:308–17. doi: 10.1111/cei.12782
201. Funk RS, Chan MA, Becker ML. Cytokine biomarkers of disease activity and therapeutic response after initiating methotrexate therapy in patients with juvenile idiopathic arthritis. Pharmacotherapy. (2017) 37:700–11. doi: 10.1002/phar.1938
202. Hong SD, Reiff A, Yang H-T, Migone T-S, Ward CD, Marzan K, et al. B lymphocyte stimulator expression in pediatric systemic lupus erythematosus and juvenile idiopathic arthritis patients. Arthritis Rheum. (2009) 60:3400–9. doi: 10.1002/art.24902
203. Ota Y, Niiro H, Ota S-I, Ueki N, Tsuzuki H, Nakayama T, et al. Generation mechanism of RANKL(+) effector memory B cells: relevance to the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. (2016) 18:67. doi: 10.1186/s13075-016-0957-6
204. Sarma PK, Misra R, Aggarwal A. Elevated serum receptor activator of NFkappaB ligand (RANKL), osteoprotegerin (OPG), matrix metalloproteinase (MMP)3, and ProMMP1 in patients with juvenile idiopathic arthritis. Clin Rheumatol. (2008) 27:289–94. doi: 10.1007/s10067-007-0701-3
205. Agarwal S, Misra R, Aggarwal A. Synovial fluid RANKL and matrix metalloproteinase levels in enthesitis related arthritis subtype of juvenile idiopathic arthritis. Rheumatol Int. (2009) 29:907–11. doi: 10.1007/s00296-008-0805-3
206. Lien G, Ueland T, Godang K, Selvaag AM, Førre OT, Flatø B. Serum levels of osteoprotegerin and receptor activator of nuclear factor -κB ligand in children with early juvenile idiopathic arthritis: a 2-year prospective controlled study. Pediatr Rheumatol Online J. (2010) 8:30. doi: 10.1186/1546-0096-8-30
207. Harris JG, Kessler EA, Verbsky JW. Update on the treatment of juvenile idiopathic arthritis. Curr Allergy Asthma Rep. (2013) 13:337–46. doi: 10.1007/s11882-013-0351-2
208. Blazina Š, Markelj G, Avramovič MZ, Toplak N, Avčin T. Management of juvenile idiopathic arthritis: a clinical guide. Paediatr Drugs. (2016) 18:397–412. doi: 10.1007/s40272-016-0186-0
209. Vanoni F, Minoia F, Malattia C. Biologics in juvenile idiopathic arthritis: a narrative review. Eur J Pediatr. (2017) 176:1147–53. doi: 10.1007/s00431-017-2960-6
210. Cimaz R, Marino A, Martini A. How I treat juvenile idiopathic arthritis: a state of the art review. Autoimmun Rev. (2017) 16:1008–15. doi: 10.1016/j.autrev.2017.07.014
211. Nieto-González JC, Monteagudo I. Intra-articular joint injections in juvenile idiopathic arthritis: state of the art. Reumatol Clin. (2019) 15:69–72. doi: 10.1016/j.reumae.2018.07.003
212. Filosco F, Giallongo A, Leonardi S, Tomaselli V, Barone P. Early intra-articular corticosteroid injection is predictors of remission of juvenile idiopathic arthritis. Minerva Pediatr. (2021). doi: 10.23736/S2724-5276.21.06343-6. [Epub ahead of print].
213. Kimura Y, Fieldston E, Devries-Vandervlugt B, Li S, Imundo L. High dose, alternate day corticosteroids for systemic onset juvenile rheumatoid arthritis. J Rheumatol. (2000) 27:2018–24.
214. Ringold S, Weiss PF, Beukelman T, DeWitt EM, Ilowite NT, Kimura Y, et al. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum. (2013) 65:2499–512. doi: 10.1002/art.38092
215. Batu ED. Glucocorticoid treatment in juvenile idiopathic arthritis. Rheumatol Int. (2019) 39:13–27. doi: 10.1007/s00296-018-4168-0
216. van Rossum MAJ, van Soesbergen RM, Boers M, Zwinderman AH, Fiselier TJW, Franssen MJAM, et al. Long-term outcome of juvenile idiopathic arthritis following a placebo-controlled trial: sustained benefits of early sulfasalazine treatment. Ann Rheum Dis. (2007) 66:1518–24. doi: 10.1136/ard.2006.064717
217. Ferrara G, Mastrangelo G, Barone P, La Torre F, Martino S, Pappagallo G, et al. Methotrexate in juvenile idiopathic arthritis: advice and recommendations from the MARAJIA expert consensus meeting. Pediatr Rheumatol Online J. (2018) 16:46. doi: 10.1186/s12969-018-0255-8
218. Ayaz NA, Karadag SG, Çakmak F, Çakan M, Tanatar A, Sönmez HE. Leflunomide treatment in juvenile idiopathic arthritis. Rheumatol Int. (2019) 39:1615–9. doi: 10.1007/s00296-019-04385-7
219. Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. (2000) 342:763–9. doi: 10.1056/NEJM200003163421103
220. Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. (2008) 359:810–20. doi: 10.1056/NEJMoa0706290
221. Horneff G, Ebert A, Fitter S, Minden K, Foeldvari I, Kümmerle-Deschner J, et al. Safety and efficacy of once weekly etanercept 08 mg/kg in a multicentre 12 week trial in active polyarticular course juvenile idiopathic arthritis. Rheumatology. (2009) 48:916–9. doi: 10.1093/rheumatology/kep122
222. Ruperto N, Lovell DJ, Cuttica R, Woo P, Meiorin S, Wouters C, et al. Long-term efficacy and safety of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis: findings from an open-label treatment extension. Ann Rheum Dis. (2010) 69:718–22. doi: 10.1136/ard.2009.100354
223. Brunner HI, Ruperto N, Tzaribachev N, Horneff G, Chasnyk VG, Panaviene V, et al. Subcutaneous golimumab for children with active polyarticular-course juvenile idiopathic arthritis: results of a multicentre, double-blind, randomised-withdrawal trial. Ann Rheum Dis. (2018) 77:21–9. doi: 10.1136/annrheumdis-2016-210456
224. Ruperto N, Lovell DJ, Quartier P, Paz E, Rubio-Pérez N, Silva CA, et al. Long-term safety and efficacy of abatacept in children with juvenile idiopathic arthritis. Arthritis Rheum. (2010) 62:1792–802. doi: 10.1002/art.27431
225. Lovell DJ, Ruperto N, Mouy R, Paz E, Rubio-Pérez N, Silva CA, et al. Long-term safety, efficacy, and quality of life in patients with juvenile idiopathic arthritis treated with intravenous abatacept for up to seven years. Arthritis Rheumatol. (2015) 67:2759–70. doi: 10.1002/art.39234
226. Calvo-Río V, Santos-Gómez M, Calvo I, González-Fernández MI, López-Montesinos B, Mesquida M, et al. Anti-interleukin-6 receptor tocilizumab for severe juvenile idiopathic arthritis-associated uveitis refractory to anti-tumor necrosis factor therapy: a multicenter study of twenty-five patients. Arthritis Rheumatol. (2017) 69:668–75. doi: 10.1002/art.39940
227. Ramanan AV, Dick AD, Guly C, McKay A, Jones AP, Hardwick B, et al. Tocilizumab in patients with anti-TNF refractory juvenile idiopathic arthritis-associated uveitis (APTITUDE): a multicentre, single-arm, phase 2 trial. Lancet Rheumatol. (2020) 2:e135–41. doi: 10.1016/S2665-9913(20)30008-4
228. Maleki A, Manhapra A, Asgari S, Chang PY, Foster CS, Anesi SD. Tocilizumab employment in the treatment of resistant juvenile idiopathic arthritis associated uveitis. Ocul Immunol Inflamm. (2021) 29:14–20. doi: 10.1080/09273948.2020.1817501
229. Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. (2012) 367:2396–406. doi: 10.1056/NEJMoa1205099
230. Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat NM, Horneff G, et al. Canakinumab in patients with systemic juvenile idiopathic arthritis and active systemic features: results from the 5-year long-term extension of the phase III pivotal trials. Ann Rheum Dis. (2018) 77:1710–9. doi: 10.1136/annrheumdis-2018-213150
231. Brunner HI, Quartier P, Alexeeva E, Constantin T, Koné-Paut I, Marzan K, et al. Efficacy and safety of canakinumab in patients with systemic juvenile idiopathic arthritis with and without fever at baseline: results from an open-label, active-treatment extension study. Arthritis Rheumatol. (2020) 72:2147–58. doi: 10.1002/art.41436
232. Nigrovic PA, Mannion M, Prince FHM, Zeft A, Rabinovich CE, van Rossum MAJ, et al. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum. (2011) 63:545–55. doi: 10.1002/art.30128
233. Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C, et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial). Ann Rheum Dis. (2011) 70:747–54. doi: 10.1136/ard.2010.134254
234. Lovell DJ, Giannini EH, Reiff AO, Kimura Y, Li S, Hashkes PJ, et al. Long-term safety and efficacy of rilonacept in patients with systemic juvenile idiopathic arthritis. Arthritis Rheum. (2013) 65:2486–96. doi: 10.1002/art.38042
235. Ilowite NT, Prather K, Lokhnygina Y, Schanberg LE, Elder M, Milojevic D, et al. Randomized, double-blind, placebo-controlled trial of the efficacy and safety of rilonacept in the treatment of systemic juvenile idiopathic arthritis. Arthritis Rheumatol. (2014) 66:2570–9. doi: 10.1002/art.38699
236. Huang Z, Lee PY, Yao X, Zheng S, Li T. Tofacitinib treatment of refractory systemic juvenile idiopathic arthritis. Pediatrics. (2019) 143:e20182845. doi: 10.1542/peds.2018-2845
237. Miserocchi E, Giuffrè C, Cornalba M, Pontikaki I, Cimaz R. JAK inhibitors in refractory juvenile idiopathic arthritis-associated uveitis. Clin Rheumatol. (2020) 39:847–51. doi: 10.1007/s10067-019-04875-w
238. Ruperto N, Brunner HI, Synoverska O, Ting TV, Mendoza CA, Spindler A, et al. Tofacitinib in juvenile idiopathic arthritis: a double-blind, placebo-controlled, withdrawal phase 3 randomised trial. Lancet. (2021) 398:1984–96. doi: 10.1016/S0140-6736(21)01255-1
239. Ramanan AV, Guly CM, Keller SY, Schlichting DE, de Bono S, Liao R, et al. Clinical effectiveness and safety of baricitinib for the treatment of juvenile idiopathic arthritis-associated uveitis or chronic anterior antinuclear antibody-positive uveitis: study protocol for an open-label, adalimumab active-controlled phase 3 clinical trial (JUVE-BRIGHT). Trials. (2021) 22:689. doi: 10.1186/s13063-021-05651-5
240. Welzel T, Winskill C, Zhang N, Woerner A, Pfister M. Biologic disease modifying antirheumatic drugs and Janus kinase inhibitors in paediatric rheumatology—what we know and what we do not know from randomized controlled trials. Pediatr Rheumatol Online J. (2021) 19:46. doi: 10.1186/s12969-021-00514-4
241. Minden K. Adult outcomes of patients with juvenile idiopathic arthritis. Horm Res. (2009) 72(Suppl.1):20–5. doi: 10.1159/000229759
242. Selvaag AM, Aulie HA, Lilleby V, Flatø B. Disease progression into adulthood and predictors of long-term active disease in juvenile idiopathic arthritis. Ann Rheum Dis. (2016) 75:190–5. doi: 10.1136/annrheumdis-2014-206034
243. Edwards JCW, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. (2004) 350:2572–81. doi: 10.1056/NEJMoa032534
244. Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. (2006) 54:1390–400. doi: 10.1002/art.21778
245. Kuek A, Hazleman BL, Gaston JH, Ostör AJK. Successful treatment of refractory polyarticular juvenile idiopathic arthritis with rituximab. Rheumatology. (2006) 45:1448–9. doi: 10.1093/rheumatology/kel301
246. Narváez J, Díaz-Torné C, Juanola X, Geli C, Llobet JM, Nolla JM, et al. Rituximab therapy for refractory systemic-onset juvenile idiopathic arthritis. Ann Rheum Dis. (2009) 68:607–8. doi: 10.1136/ard.2008.092106
247. Feito JG, Pereda CA. Rituximab therapy produced rapid and sustained clinical improvement in a patient with systemic onset juvenile idiopathic arthritis refractory to TNF alpha antagonists. J Clin Rheumatol. (2009) 15:363–5. doi: 10.1097/RHU.0b013e3181ba3c6f
248. Alexeeva EI, Valieva SI, Bzarova TM, Semikina EL, Isaeva KB, Lisitsyn AO, et al. Efficacy and safety of repeat courses of rituximab treatment in patients with severe refractory juvenile idiopathic arthritis. Clin Rheumatol. (2011) 30:1163–72. doi: 10.1007/s10067-011-1720-7
249. Jansson AF, Sengler C, Kuemmerle-Deschner J, Gruhn B, Kranz AB, Lehmann H, et al. B cell depletion for autoimmune diseases in paediatric patients. Clin Rheumatol. (2011) 30:87–97. doi: 10.1007/s10067-010-1630-0
250. Stoll ML, Cron RQ. Treatment of juvenile idiopathic arthritis: a revolution in care. Pediatr Rheumatol Online J. (2014) 12:13. doi: 10.1186/1546-0096-12-13
251. Sakamoto AP, Pinheiro MM, Barbosa CMPL, Fraga MM, Len CA, Terreri MT. Rituximab use in young adults diagnosed with juvenile idiopathic arthritis unresponsive to conventional treatment: report of 6 cases. Rev Bras Reumatol. (2015) 55:536–41. doi: 10.1016/j.rbr.2014.12.015
252. Reis J, Aguiar F, Brito I. Anti CD20 (Rituximab) therapy in refractory pediatric rheumatic diseases. Acta Reumatol Port. (2016) 41:45–55.
253. Kearsley-Fleet L, Sampath S, McCann LJ, Baildam E, Beresford MW, Davies R, et al. Use and effectiveness of rituximab in children and young people with juvenile idiopathic arthritis in a cohort study in the United Kingdom. Rheumatology. (2019) 58:331–5. doi: 10.1093/rheumatology/key306
254. McAtee CL, Lubega J, Underbrink K, Curry K, Msaouel P, Barrow M, et al. Association of rituximab use with adverse events in children, adolescents, and young adults. J Am Med Assoc Netw Open. (2021) 4:e2036321. doi: 10.1001/jamanetworkopen.2020.36321
Keywords: juvenile idiopathic arthritis (JIA), B cells, autoantibodies, inflammation, immunopathogenesis
Citation: Moura RA and Fonseca JE (2022) B Cells on the Stage of Inflammation in Juvenile Idiopathic Arthritis: Leading or Supporting Actors in Disease Pathogenesis? Front. Med. 9:851532. doi: 10.3389/fmed.2022.851532
Received: 10 January 2022; Accepted: 09 February 2022;
Published: 04 April 2022.
Edited by:
Tadej Avcin, University Medical Centre Ljubljana, SloveniaCopyright © 2022 Moura and Fonseca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rita A. Moura, ritaaguiarmoura@gmail.com
 Rita A. Moura
Rita A. Moura João Eurico Fonseca
João Eurico Fonseca