

Most atoms are stable but some unstable atoms break apart and release particles (alpha, beta and neutron) and electromagnetic waves (gamma rays). This process is called radioactivity. The particles and energy released is radiation.
Background radiation is around us all the time. It comes from many sources, including the ground, the sun, and the food we eat and drink. Many substances, both natural and man-made, are radioactive.
There are many types of radiation. The table below shows the main types of radiation we need to manage in the nuclear sector.
| Radiation type | Details | Shielding |
| α
Alpha (α)Particles |
|
Paper, clothing or the skin |
| β
Beta (β)Particles |
|
Thin aluminium or lead |
| γ
Gamma (γ)Waves (Part of electromagnetic spectrum) |
|
Several centimetres of lead or metres of concrete |
| n
Neutron Particles |
|
Several metres of concrete or water |
The amount of radioactivity given off by a radioactive substance decreases over time. This is called radioactive decay.

The time it takes for the amount of radioactivity to decrease by 50% is called the half-life. Different radioactive atoms have different half-lives: some can be a matter of seconds while some can be many thousands of years.
Radioactive substances with a short half-life are highly radioactive, producing a lot of radiation but not for very long. Radioactive substances with a long half-life are generally less radioactive, but emit radiation for a long time.
Background radiation is around us all the time. It comes from many sources, including the ground, the sun and the food we eat and drink.
The total amount of radiation we experience day-to-day is low. On average, about 84% of background radiation is from natural sources and 15% from medical practices, such as X-rays. Less than 1% comes from nuclear power, industrial and defence activities.
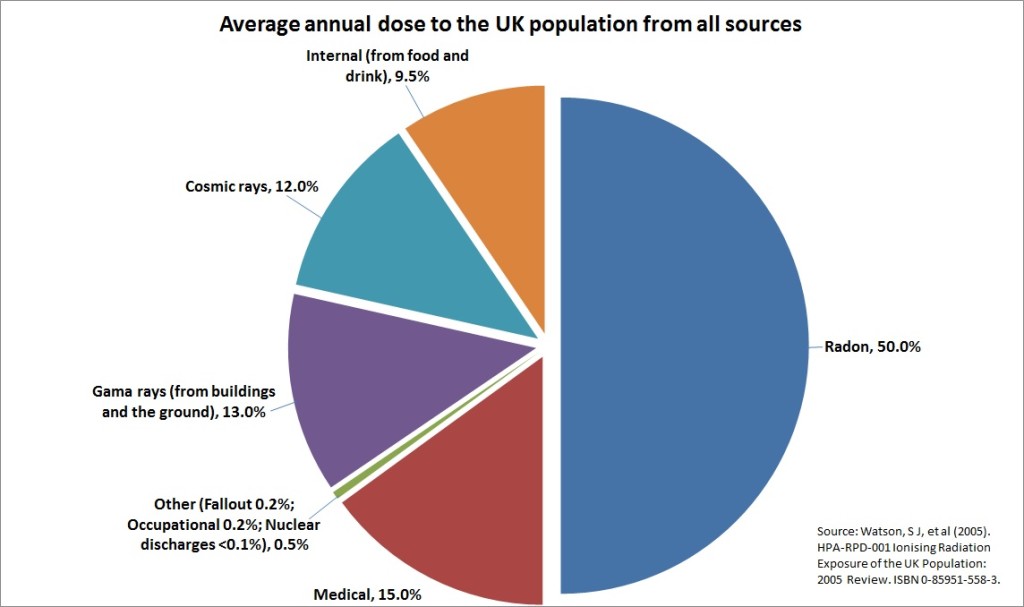
In the UK, about half of the radiation we receive comes from natural radon gas. Radon gas is produced from the decay of natural uranium found in rocks and soils in the ground. To find out more about radon gas in the UK, visit the Public Health England ‘UKradon’ website.
Radioactive materials have many uses in industry, agriculture, medicine and research.
We use radioactive materials to:
Find out more about working with radiation from the Health and Safety Executive
Radiation can be harmful to human health and the environment if it is not properly controlled. The amount of harm caused depends on the radiation dose received. This dose depends on the radiation type, the radiation’s energy and how much reaches the person or the environment.
A radiation dose can be received by eating or breathing in radioactive materials or by being exposed to external radiation. A consistent dose of radiation can increase the risk of cancer later in life. A very high dose acts like a poison and can be fatal. The use of radioactive materials is tightly controlled and a number of protective measures are used to prevent exposure to high doses. This includes legislation to limit exposure and physical barriers to sources of high radiation.
The total amount of radiation we experience day-to-day is low. The table below shows the approximate radiation dose from different sources of exposure. The radiation dose is measured in milliSievert (mSv).
The legal exposure limit for nuclear industry employees is 20mSv per year, although the typical occupational exposure is over 100 times less than the legal limit. Negative health effects can be observed with doses above 100mSv.
Find out more about the legal exposure limits from the Health and Safety Executive
| Source of Exposure | Radiation Dose (mSv) |
| Dental x-ray | 0.005 |
| 100g of Brazil nuts | 0.010 |
| Chest x-ray | 0.014 |
| Transatlantic flight | 0.08 |
| Average annual occupational exposure for a nuclear power station worker (2010) | 0.18 |
| UK annual average dose from natural radon gas | 1.3 |
| UK average annual radiation dose | 2.7 |
| CT scan of the chest | 6.6 |
| Average annual dose to people in Cornwall from natural radon gas | 7.8 |
| Annual exposure limit for nuclear industry employees | 20 |
| Level at which changes in blood cells can be readily observed | 100 |
| Acute radiation effects observed, including nausea and a reduction in white blood cell count | 1000 |
| Dose of radiation which would kill about half of those receiving it in a month | 5000 |
Source: Public Health England (2011)
Regulations control the storage, use and disposal of radioactive substances, helping to protect people and the environment.

Some radioactive substances need to be carefully controlled to protect people and the environment.
Find out more about how the storage, use and disposal of radioactive substances are regulated
Download our factsheets to learn more about radioactive waste