9. Inspection and testing
The meaning of log cfu/g or cfu/ml, and how to read micro lab reports
Micro results don’t need to be daunting, here are the basics you need to know.

The standards
This article walks you through the steps of product inspection, testing and the environmental monitoring programme. The following information complies with:
| BRCGS Food Safety Issue 9 | 5.6.2 Understanding results |
| BRCGS Packaging Issue 6 | 4.8.5 Understanding results |
| BRCGS Agents & Brokers Issue 3 |
4.4.5 Understanding results |
| Storage & Distribution Issue 4 | No specific references |
| FSSC22000 Version 5.1 | No specific references |
| IFS Food Version 7 | 5.6.4 Analysis of results |
| SQF Edition 9 | No specific references |
Please note, that although some of the above standards don’t mention specifically that you must understand the results of testing, they all do expect corrective and preventive actions to be taken. Which indirectly means, you must understand the results – in order to apply the right type of action.
Important for the pharma industry: This article is written for the food industry. We get a lot of questions about pharmaceuticals, which we will try to answer but as we are not specialists in this field. The reference standards are different for pharmaceuticals and are therefore not something we’re familiar with.
The requirements
The significance of the laboratory results must be understood, so the appropriate action can be taken.
Staff responsible for reading and interpreting results, must be able to prove that they’re competent.
BRCGS Food Safety Issue 9
In BRCGS Food Safety Issue 8 a new requirement was added, which meant that sites must be able to understand and interpret the results of laboratory results. In BRCGS Food Safety Issue 9 this requirement was reiterated, as it was added to the interpretation of the clause.
For Issue 9 the clause has also been expanded, to state that the site must understand the measurement uncertainty used by the lab and how it’s been applied, unless this is defined by legislation in the given country.
How to read a micro lab report
Unless you’ve worked in a lab or had to manage one, being faced with a micro lab report can be daunting.
Unfortunately, there’s no simple training courses you can go on where they teach you how to read a lab report. In this article we’re going to take you through all the key points you need to know, so you can read micro lab reports with ease.
You might see the following types of results on a microbial lab report:
- Detected or Not Detected
- A specific result (e.g. 100cfu/g)
- A less than result (e.g. <10cfu/g)
- A log result (e.g. 105cfu/g)
We’ll go through each type of result, so you understand each one.
1. Detected or Not Detected
This type of result is given to pathogenic bacteria that only need a small quantity of bacteria to be present in the food, to cause food poisoning – such as Listeria or Salmonella.
Therefore, the lab will report that they have either detected the bacteria (reported as ‘D’) or not detected the bacteria (reported as ‘ND’).
2. A specific result (e.g. 100cfu/g)
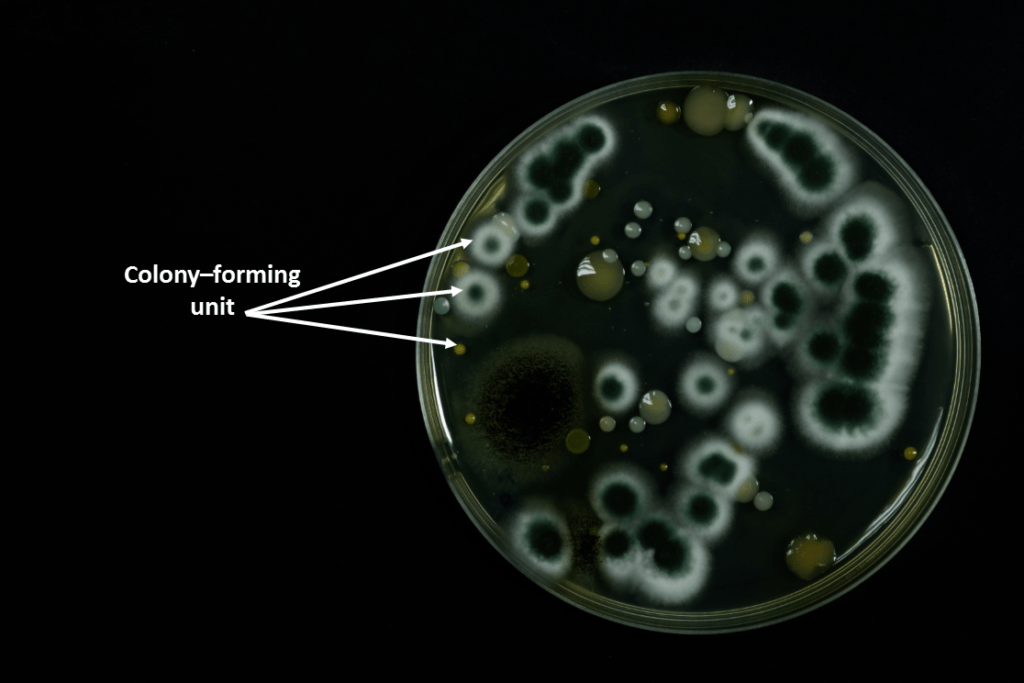
Results like this are either provided in cfu/g or cfu/ml. cfu stands for colony-forming unit. This means that cfu/g is colony-forming unit per gram and cfu/ml is colony-forming unit per millilitre.
A colony-forming unit is where a colony of microbes grow on a petri dish, from one single microbe. On the petri dish image below, each one of these would be a colony-forming unit.
When the lab carries out your test, they count of the number of colony-forming units on the petri dish. They give you the results of the number of colony-forming units, for the number of grams or millilitres of test material that they put on the petri dish.
So, in the most simplistic terms this would be, for example:
- 100 colony-forming units for one gram put on the petri dish.
- This would be written as: 100cfu/g.
3. A less than result (e.g. <10cfu/g)
Sometimes you get results that say: <10cfu/g or <100cfu/g. But if you think about it, you can’t get less than 1 colony-forming unit on a petri dish – can you? It’s either there or it’s not.
What this means is, that the lab hasn’t seen any colony-forming units on the petri dish at all. But they can’t say that the result is ‘0’ (zero), as every test has an inaccuracy. By reporting <10cfu/g, the lab is saying that there’s an inaccuracy of 10 in that test, but essentially that the result was 0, because they couldn’t find anything on the plate.
4. A log result (e.g. 105cfu/g)
When a result is high, the result is condensed using what are called logarithms. The logarithm is the superscript number to the right of the 10.
To be able to read logarithms, you need understand how they work.
Understanding logarithms
A micro logarithm is generally known as a ‘log.’ The log is a value of how many cfu (colonies) there are. Each log is worth a multiple of 10, as follows:
Logs start at 1. Each log increase, increases the cfu by a multiple of 10. One easy way of remembering this is that you add the log number of zeros to 1 to create the log.
Table 1
| Log | cfu number |
|---|---|
| 1 | 10 |
| 2 | 100 |
| 3 | 1,000 |
| 4 | 10,000 |
| 5 | 100,000 |
Table 2
| Log | cfu number |
|---|---|
| 1 | 10 to 99 |
| 2 | 100 to 999 |
| 3 | 1,000 to 9,999 |
| 4 | 10,000 to 99,999 |
| 5 | 100,000 and so on |
There’s a range of numbers in every log, shown in the table 2.
This means that if you had a result of 1,600cfu, this would be 3 log.
As it would sit within the 3 log range of between 1,000 and 9,999.
Frequently asked questions
What does log cfu/g mean?
A log is a multiple of 10, that’s used for reporting micro. cfu/g means colony-forming unit per gram. It’s basically, the number of colonies counted on a petri dish.
What is ‘D’ and ‘ND’ on my lab report?
The lab has reported that they have either detected the bacteria (reported as ‘D’) or not detected the bacteria (reported as ‘ND’).
What’s a less than result mean?
What this means is, that the lab has not seen any colony-forming units on the petri dish at all. But they can’t say that the result is ‘0’ (zero), as every test has an inaccuracy. By reporting <10cfu/g, the lab is saying that there’s an inaccuracy of 10 in that test, but essentially that the result was 0, because they couldn’t find anything on the plate.
What’s a greater than result mean?
You’ll get a greater than result, if the results are really high. When this happens and the plate is too full to count. This means, that the lab can only tell you the maximum number of colony-forming units that they would have been able to count on the plate and say that it was greater than that.
What do I need to do if my sample is expected to have a high count?
Ask the lab to dilute your sample so you can get an accurate count.
What size area should I swab if the lab hasn’t specified this?
Any size is OK as long as you record the size of the area that you’ve swabbed so you can interpret the result when you get it back.
Why are logarithms used by the lab when they send the results back to me?
The count on the plate you sent was high, so they’ve shown the result using a log count to reduce the number of zero’s shown in the result.
Have your say…
47 thoughts on “The meaning of log cfu/g or cfu/ml, and how to read micro lab reports”
Share your thoughts…
We've tagged this article as: inspection and testing, Microbiology, validation


When plotting CFU values in a survival series some of the CFU values are 0, what would be a suitable way to plot these on a scatter graph (Log CFU/ml on Y and Time on X). Can you Leave the values of 0 as 0 and log the rest (mention in legend this is whats done?)
Hi James
You could. You could also set the minimum as -1 so that you can ‘see’ the 0 results?
Kassy
Hi
I have a question.
If i have 0,5 log cfu, does it mean i have something lika 0,5 cfu/g ?
I know it is under the 10 cfu from your explaination, but as it i mentioned in a law and i need to be able to expalin what that 0,5 log cfu value means.
Sorry Ambre, I don’t understand the question. I would need some context please?
what does 73.3 cfu/g means??
and what does <1.1 cfu/g means??
i want to know if these results anre safe to consume ? my product is sweet potato peel polvoron
Hi Jovanne
I can’t comment on your particular set of results I’m afraid, you need to be able to understand your own results.
We have a course that will help you to understand them: https://techni-k.co.uk/shop/training/mini-trainings/understanding-laboratory-results/
Kassy
Hello!
We received test results for our water, and it is 24 cfu/ml. We give this water to livestock. Is it safe for them? What is an acceptable level? Thanks!
Hi Brynn
Here is a link to the WHO water guidelines: https://techni-k.co.uk/legislation/food-industry-legislation/#water
These are for the humans, I’m not aware of any for livestock I’m afraid.
Kassy
Hello Kassy,
Thanks for the article. Usually some labs give the results as >3000 cfu/g or something like that. So what does this > exactly denotes. Is it between 3000 to 4000 or should I consider it 3000-10000?
Thank you!
Hi,
The symbol “>” means “more than”. So in this instance it means more than 3000cfu/g.
Kassy
Hi Kassy,
thanks for the article, I need your help to understand the below result for the food company:
Compound/Analyte Method. LOR Units S1433358/1
Coliforms M8.8 – AOAC 991.14. 10 CFU/g 50000
Standard Plate Count M2.5 – AOAC 990.12 10 CFU/g. 40000000
Hi Adullah
Unfortunately I can’t help you with your results, as I have no context. I’d need to know what the material was that you were testing, why you were testing it, where it was in it’s shelf life (if relevant) and the list goes on.
We have a mini training that is designed to help you read micro results, which I think would really help you:https://techni-k.co.uk/shop/training/mini-trainings/understanding-laboratory-results/
Kassy
Hi!
For example, why the maximum acceptable count in 10¹ is 200 UFC and not 100UFC? I see it’s due to the logarithmic but I don´t know how to explain it properly if someone asks me.
Hi Maria,
Given that you’re using the term ‘UFC’ I think you must be in the pharmaceutical industry. We are only referring to food safety standards in this article and therefore I can’t answer your query I’m afraid.
Kassy
Hi..can u help interpret for me result of 9.6log cfu/g ?..how much is it?
9.6 x 1 log? How much do you think it would be, given the information from the article?
Hi. Some help needed please. I’ve read the article and have learnt a bit. I got my results back yesterday from testing, but don’t understand the one result.
It states Food/ Total plate count 1680 is this good or not?
Hi Bev,
Unfortunately I can’t say – as it depends on what you’re testing. What does the specification you’re working to say?
Kassy
In which case maximum acceptable count is allowed during interpretation of microbial limit test of non sterile products?
Hi Dharmen
I’m sorry I don’t understand the question. Can you try rephrase it for me?
Kassy
A very useful article. I would love to see you write an article on Ink Migration Testing and its mysteries. Many thanks
That brightened my day Gerry! One day I’ll get to it I’m sure… 🙂
what is aerobic and anaerobic ?please
Aerobic means that ‘air’ is needed. Anaerobic, means ‘air’ isn’t needed.
who can help me with interpretation “The aerobic mesophilic bacteria (AMB) density varied from 2.9 to 9.5 Log10 CFU/g”
How much is it 9.5 Log10 CFU/g ?
Hi Anastasija
Sounds like this is more than one test result. You’d need to speak to your lab to clarify.
Thanks
Kassy
What is an estimated result for E. Coli and Coliforms mean
It means that the lab isn’t sure of the exact result. This could be for many reasons, you’d need to speak to your lab to find out the cause.
Thanks
Kassy
Thanks for this article. I have a question on microbial limits that confuse me. If one were to compare a specification:
ND in 1g
ND in 10g
ND in 25g
Which is the better specification?
Hi Mike
ND in 1g would be more accurate – if that’s what you mean? Out of interest – why do you ask?
Kassy
What about there’s no dilution, like water testing in micro. As in 1ml water pipette in petridish and then it pour agar, then after 3 days there’s no growth or no bacteria. What is the data result? Its 0 cfu/ml or <1 cfu/ml or <10 cfu/ml ?
Hi Em,
Where there’s no dilution and no growth, then it’s
Very helpful.
Firstly, thank you for the explanation
My question is how do you convert cfu/mL to cfu/g?
I have a liquid sample diluted 13 times, taken 1mL for plating and got the count as 20.8×10^13 cfu/mL
So for converting it to cfu/g, should I substitute the volume 1mL to 1g and calculate or use the density of the sample or media and then use the resulted mass in the cfu/mg formula
Hi,
Why would you want to convert it? Surely a liquid sample wouldn’t make sense being presented in grams?
Kassy
you explain the concept very good. thank.
This is a great article, thank you for this. It is so easy to forget how to interpret the results at times, but even harder to actually explain to people what the results mean and why. This article clears that up. I’ve already sent the link to my Technical Team and asked them to read it in preparation for an on-site workshop on the subject.
Another excellent article. Thank you for sharing.
MPN is generally used in liquid samples like water, the lab we use has this method for water samples we send in.
This article gives a pretty plain language explanation. Its a bit more of an estimate than other methods, but it has its advantages.
https://microbenotes.com/water-quality-analysis-by-most-probable-number-mpn/
Thanks Rebecca!
Great article thanks.
Can you explain MPN and how does this relate to cfu?
Hi Paul
MPN isn’t something I’ve heard of to be honest. In what context have you seen it?
Kassy
MPN isn’t a result that would ordinarily be provided on a certificate for the related tests we do in the food industry, such as tests on food or environmental. It certainly isn’t a measure I have come across.
MPN, or Most Proabable Number, is a calculation based on liquid broth growth using 10-fold dilutions of the sample. It works, in essence, in a similar format to counting cfu on a petri dish.
If you’re expecting high results, and you have asked the lab to dilute the sample accordingly, then MPN may be reported instead of cfu. However, it would have to be a very specific type of product for this.
This article I found online gives some interesting information on the use of MPN: https://microbeonline.com/probable-number-mpn-test-principle-procedure-results/
Well and clearly explained. Thank you
Handy briefing article!
We’ve last month issued (with FSS) “Guidance for Food Business Operators: Getting the Best from Third Party Laboratories”.
This Guidance aims to raise FBOs’ awareness of the need to use analytical laboratories with the right expertise, accreditations, using appropriate methods and facilitate development of partnerships between such third-party laboratories and their customers in the food industry, moving away from purely transactional arrangements.
The focus of this first edition is on microbiological analytical services provided by a third party to a FBO.
The Guidance is a free download (https://www.chilledfood.org/guidance-for-food-business-operators-getting-the-best-from-third-party-laboratories) and includes:
Checklist
Fitness for Purpose – Laboratories and Methods
Provision of Samples to Laboratories
Reporting Results
Complaints Procedure
Selecting a laboratory through tender
Special measures for laboratories
ContractsAppendices
1. Terminology
2. Microbiology
2.1 Legally Recognised Methods
2.2 What other Microbiological Tests are Relevant for Various Food Materials
Tables
1: Industrywide Continuous Improvement Indicators for Laboratories
2: Findings, Laboratory Action and Communication of Results
3: Microbiological Methods – Specified by EU Regulation 2073/2005
4: Typical Expected Turnaround of Microbiological Tests if Compliant with Standard Methods
Brill, thank you so much for sharing Karin!
A simple and good article to read.