Abstract
Purpose
Home-made alcohol-related deaths are a significant public health issue which is often overlooked. To date, approximately 30 people have died following the consumption of home brewed alcoholic beverages in Botswana. The process of brewing such alcoholic beverages remains unmonitored and makes it difficult to pinpoint the cause of these deaths. Ethanol content in these beverages is thought to be high and therefore contributing towards the deaths. The aim of this research was to develop and validate a method for the quantification of ethanol in the different types of home-brewed alcoholic drinks of Botswana.
Methods
Twenty-six different samples of home-brewed alcoholic drinks were collected from local brewers in different districts of Botswana. A Liquid–Liquid Extraction-Gas Chromatography-Flame Ionization Detector method was optimized for extraction of ethanol using ethyl acetate and validated for accuracy, precision, repeatability, selectivity, linearity, limit of detection, limit of quantification, stability. Following this, the method used to measure the concentration of ethanol in the different home brewed beverages.
Results
The method demonstrated linearity in the concentration range of 2.5–60% v/v with correlation coefficient (R2) of 0.996 and, was found to be precise with %RSD values ≤ 5%. Repeatability was acceptable with %RSD values ≤ 5%. Percentage recoveries were within 100%. No interference was observed from likely excipients commonly found in home brewed alcoholic beverages, demonstrating good selectivity. Limits of detection and quantification were found to be 0.37% v/v and 1.12% v/v respectively. The analysed samples were discovered to contain ethanol with concentrations ranging from 2.56 to 36% v/v.
Conclusion
A method for the quantification of ethanol in home-made alcoholic beverages of Botswana was developed and validated. It is simple, cheap, rapid and does not require sophisticated instruments.
Similar content being viewed by others
1 Introduction
In different parts of the world, varieties of alcoholic beverages are home brewed and consumed [1]. Indigenous fermented alcoholic beverages from different parts of the world have been described [2]. These include some fruit based spirits of brandy collectively known as Rakija or brija which are consumed in East European countries [3], Kefir or kefyr, produced from a combination of milk and kefir grains or from water, dried fruits and lemon which is common in Central Asia and Middle East [4,5,6], moonshine in India, Sima; a sucrose-based alcoholic drink consumed in Finland as well as Ayran and Boza (produced from millet or wheat) and widely consumed in Turkey [7, 8]. In Africa, there is tella in Ethiopia, thobwa in Malawi, burukutu in Nigeria, tonto in Uganda, muratina in Kenya, as well as chibuku in Zimbabwe and Umqombothi in South Africa [9,10,11,12].
Traditional alcoholic beverages are relatively cheap to prepare and are important alternatives for low-income consumers who cannot afford imported or industrially processed beverages [13, 14]. Brewers prepare traditional alcoholic beverages for market, weddings, ceremonies, and other celebrations and to generate income for their daily needs. Some households generate enough income from the sale of these beverages to pay for their children's education, put food on the table and meet all the family’s basic needs [15,16,17]. There has been growing concerns about the hygienic handling of raw materials, production procedures and qualities of the final products including the rise in alcoholism, regarding home brewed alcoholic beverages in African [18, 19]. Considerable evidence demonstrates that some beverages are contaminated with bacteria and also contain harmful impurities and adulterants [20, 21].
Oduori et al. reported that bacteria types such as Staphylococcus, Escherichia, and Salmonella were found in several traditional food products and beverages in Africa [22]. In extreme cases, death might result, as was the case in South Africa in June 2022 where 21 teenagers died. In June 2019, 9 American tourists died in the Dominican Republic while 12 people died in Uganda after consuming some traditional alcoholic drinks [23, 24]. Similar cases of suspected poisoning due to home brewed alcoholic beverages have been reported in Kenya and Iran [25, 26]. Like other African countries, Botswana is also experiencing a challenge of high mortality rates due the consumption of homemade alcoholic beverages [27]. In recent past, there has been cases of people dying after drinking traditional alcoholic beverages [28, 29]. Five people died in the Ghantsi district in 2017and two others died in 2019 in the Kgalagadi district [30,31,32]. It is likely that these numbers increased in 2020 during the government imposed Covid-19 pandemic lockdown when local pubs and breweries were banned from selling alcoholic beverages. Consequently, most people resorted to the consumption of alcoholic drinks which were illegally supplied or self-brewed.
Based on the information from the Botswana Police Service (summarised in Fig. 1 below), more homemade alcohol related deaths were recorded for women (56%) than for men (44%) between the years 2011 and June 2021. It is not known why this happened and, as such, more studies are needed to explain the trend. The noteworthy number of deaths related to the consumption of home-made alcoholic beverages were recorded during the Covid-19 pandemic era [33]. Twelve deaths were reported between July 2020 and July 2022. It is possible that these numbers could be higher, considering the verbal information received from some villagers that they could not report some deaths to the police as they feared being arrested. Access to pathology reports revealed that the cause of all the deaths could not be determined [33]. Literature review shows that a Blood Alcohol Concentration (BAC) of 200–400%, could lead to nausea and vomiting, hypothermia, dysarthria, amnesia, diplopia, and nystagmus [34]. Blood alcohols levels of greater than 400% have also been reported to result in respiratory depression followed by coma and even death [34]. The lack of standardisation procedures regarding preparation of alcoholic beverages in Botswana could account for the ethanol content in these beverages being high. This needs to be investigated as a starting point for the determination of the cause of homemade alcohol related deaths.
Showing the number of reported deaths related to consumption of home-made alcoholic beverages in Botswana per year from 2011 to June 2021 [33]
The existence of undocumented and unlicensed traditional home-brewed alcoholic beverages in Botswana could be attributed to their ease of availability, accessibility, and affordability. They are usually made and sold by home-based liquor traders in locations around villages locally known as Spoto or Shebeens. They are prepared using a wide range of traditional fermented raw materials such as cereals (sorghum and maize) and other crops such as watermelons and wild fruits [35]. The most common traditional alcoholic beverages are Setopoti (prepared from ripe watermelons), Morula (made using ripe marula fruits, Mokuru/bojalwa jwa Setswana (from sorghum grains) and Sekhokho/bobirwa gin (a distillate from sorghum-based beer), and khadi (from fermented Grewia flava fruits) [27]. Distilled homemade alcoholic beverages with high alcohol content have also been reported in Botswana. The alcohol content of one of these distilled beverages called tho-tho-tho is thought to be over 80% by volume) [36]. The Tho-tho-tho is distilled from a concoction of sorghum and yeast. Other varieties of alcoholic drinks, mostly brewed overnight using a yeast and sugar combination, are said to contain very high levels of alcohol and are ominously named based on their “after consumption” experience, using such names as o lala fa (sleepover), chechisa (speed), laela mmago (say your goodbyes), motse o teng godimo (heaven is home) and so forth [37]. The unmonitored brewing and sale of traditional home-brewed alcoholic beverages in Botswana compromises the quality of these products. For example, the uncontrolled level of fermentation and lack of information about the constituents could be risky to human health.
Ethanol is the most common analyte identified in forensic toxicology laboratories [38,39,40,41]. There are numerous analytical methods for determination of alcohol content from different alcoholic beverages already available worldwide [42, 43]. However, in Botswana known alcohol concentrations are only limited to commercially sold drinks and excludes the homemade types. Mapitse et al., determined the ethanol level and other volatile compounds in Khadi while Motlhanka et al. studied fermentative microbes of Khadi [44, 45]. Both studies did not involve the use of advanced techniques such as Gas Chromatography–Mass Spectrometry that are used by other researchers around the world [46,47,48]. These techniques are not commonly used in most African countries due to their high operational costs [49, 50].
This paper proposes the use of a validated LLE/GCFID analytical method for the quantification of ethanol in various traditional alcoholic beverages of Botswana. It is the first time that a validated method has been used for the quantification of ethanol in khadi and other home brewed alcoholic beverages.
Although there are SUPRAS and DES solvents which are used recently and considered friendly to the atmosphere due to their lower vapor pressures, they have been found to be partially miscible with water. Therefore, the use of such solvents was not suitable for this study because the homebrewed alcohol samples were mainly aqueous. Another disadvantage of using DESs is that they could end up in the aquatic environment since they do not completely dissolve in water. To prevent from this happening, laboratories would need to develop methods for recycling and their removal from the environment. This could become a financial burden, especially here in Africa where the budget is already stretched. The aim of this study was to develop an analysis method which is economical in all aspects, including the use of techniques that are already available in most laboratories.
2 Materials and Methods
A method developed by Chun et al. for the analysis of ethanol in blood was adopted and validated for use during this study [51].
2.1 Chemicals and Reagents
Ethanol (99.9%), Dichloromethane (99.7%), Hexane (99.9%), Acetonitrile (99.9%) and Ethyl acetate (99.9%) were all purchased from Merck Chemicals (Pty) Ltd (Germiston, South Africa). Sodium chloride (AR) and anhydrous sodium sulphate (AR) were purchased from Rochelle Chemicals & Lab Equipment (Johannesburg, South Africa).
2.2 Sampling
Twenty-six different samples of home brewed alcoholic beverages, each measuring 500 mL, were collected from the homes of local brewers in different villages in the Gantsi, Kgalagadi and Central District near Palapye. The collected samples included Morula (A-F), Setopoti (G), Bojalwa jwa Setswana (H&I), Sekhokho (J), Khadi (K-V), Ginger beer (W) and Chonchobina (X–Z). The samples were collected in glass bottles and transported in cooler boxes containing ice. Upon arrival at the laboratory, they were filtered and kept at 4 °C for an average of 3 days. Figure 2 below shows the different villages of Botswana where the samples were collected.
2.3 Gas Chromatography Conditions
An Agilent 6890 N capillary gas chromatograph coupled with an Agilent G4513A static headspace auto-sampler (5301 Stevens Creek Blvd. Santa Clara, CA 95051 United States) was used. Separation was achieved on a standard non-polar HP-5 capillary column, (30 m length, 0.32 mm i.d and 0.25 µm film thickness). Sample injections were made in a split mode using a general-purpose split/split-less liner packed with glass wool. The GC oven temperature program was started at 40 °C and held for 5 min, then increased to 100 °C at a rate of 10 °C min−1 and held for 4 min resulting in a total run time of 11 min. Sample volumes of 1 µL were injected into the instrument at a split ratio of 50:1 using helium as a carrier gas. The flow rate of the helium was set at a constant flow of 2 mL min−1. The injector and FID detector temperatures settings were 250 °C and 280 °C, respectively.
For identification purposes, a quality control standard solution of 30% v/v ethanol in ethyl acetate was prepared. Three replicate injections of the solution were performed using the above settings. Ethanol was detected at 5.943 min.
2.4 Preparation of Calibration Standards
Nine ethanol standard solutions were prepared by mixing 0.5, 0.75, 1, 2, 3, 4, 5 and 6 mL of ethanol with the required volumes ethyl acetake in separate volumetric flasks to achieve a final solution volume of 10 mL. The resultant concentration of the prepared solutions were 2.5, 5, 7.5, 10, 20, 30, 40, 50 and 60% v/v. Three replicate injections of each solution was performed and their response recorded. Data obtained from these were subjected to regression analysis using the least squares method to construct a calibration curve.
2.5 Measurement of pH of Collected Samples
A Thermo Scientific Orion Star A111 pH meter was used. A three-point calibration was done using buffers of pH 4.00, 7.00 and 10.00. After calibration, the electrode was rinsed with distilled water and dried using soft lint free tissue paper. The pH of all samples was then measured followed by rinsing and wiping of the electrode after each measurement. The measurement of sample pH was to establish if there is a relationship between the pH and alcohol content.
2.6 Liquid–Liquid Extraction Method
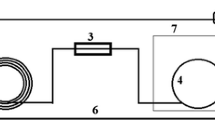
The LLE extraction technique adopted from Chun et al. was used to determine the best extraction solvent for ethanol from homemade alcoholic beverages [51]. Extraction was performed by dissolving 1 mL of home-brewed alcoholic beverage sample in 1 mL of each of the four solvents (hexane, acetonitrile, dichloromethane, and ethyl acetate). NaCl (0.5 g) was added to each solution to enhance phase separation. Following this, the solutions were vortexed for 1 min and centrifuged for 10 min at 10,000 rpm. The organic layer from each solution was removed and transferred into GC vials. A schematic representation of the extraction process is shown in Fig. 3 below. Optimization was performed to determine the sample with optimal ethanol recovery. To do this, 3 different solutions, each with a total volume of 2 mL, were prepared using sample to solvent ratios of 1:3, 1:1 and 3:1. The solvent with the most recovery was used as the working solvent for the study.
Schematic diagram of LLE process [52]
2.7 Method Validation
The method was validated as per the single laboratory validation approach of International Conference on Harmonization (ICH) Q2A and Q2B specific guidelines on method validation, which recommends the evaluation of the parameters such as selectivity, linearity, accuracy, precision, limit of quantification (LOQ), limit of detection (LOD) and robustness. Analyte quantification was based on an external standard calibration graph obtained by plotting the individual peak areas against ethanol concentrations. All samples and standards were injected in triplicates.
2.7.1 Accuracy
The accuracy of the method was achieved by comparing the results of measured ethanol values with the true values of two known commercial alcohol samples of the Bombay Sapphire (Bombay Spirits Co., Ltd, Whitchurch, RG287NR. United Kingdom) and Cheteau Del Rei-Sweet (Chill beverages, Plankenbrug, Stellenbosch 7600. South Africa), with alcohol content of 47% v/v and 7% v/v, respectively. This was achieved by injecting six replicates of each sample into the GC and comparing the results with the true values of the sample. The accuracy of the method was expressed as percent recovery.
2.7.2 Precision
There are three levels at which precision may be expressed and these are repeatability, reproducibility, and variations in the conditions for repeatability when performed within the same laboratory results with intermediate precision. For this study, the repeatability factor was used to measure precision.
2.7.2.1 Repeatability
To assess repeatability, ten replicate injections were performed for each of the two ethanol standards comprising of 5% and 30% v/v ethanol concentration. The peak areas and %RSDs were calculated across the ten injections for each standard and evaluated.
2.7.3 Selectivity
To assess the selectivity of the methods, five standard solutions comprising of likely excipients found in home-brewed alcoholic beverages were prepared in ethyl acetate. The excipients used were methanol, ethanol, acetone, 1-propanol, and 1-butanol. Each standard was first injected separately into the instrument to determine its retention time. A laboratory standard mixture was then prepared by mixing 1 mL each of the prepared standard solution. Six replicate injections of the prepared standard mixture were performed.
2.7.4 Linearity and Analytical Range
Linearity was assessesd by preparing eight sets of calibration standards of ethanol in ethyl acetate in the concentration ranges of 2.5, 5, 7.5, 10, 20, 30, 40, 50 and 60% v/v. Six replicate injections of these solutions were performed and their detector response recorded. Data obtained from these were subjected to regression analysis using the least squares method to construct a calibration curve.
2.7.5 Limit of Detection (LOD) and Limit of Quantification (LOQ)
The ICH guidelines suggest that the LOD can be determined by using the residual standard deviation (SD) of a regression line or the Standard Deviation of the Y-intercept of regression lines as a standard deviation [53]. The LOD and LOQ were derived from the standard deviation of the response and slope of the calibration curve using Eqs. 1a and 1b below.
Equation 1a LOD calculations [36, 37]
Equation 1b LOQ calculations [36, 37] where LOD = the limit of detection, LOQ = limit of quantification, SE = Standard Deviation of the analyte response and a = slope of the calibration curve for the analyte.
2.7.6 Analyte Stability
Stability was assessed through the results obtained from the analysis of two sets of in-house ethanol standard solutions with concentrations of 20% and 30% v/v and samples of morula (A) and Setopoti (G), (see Table 1 below). Analysis was performed for five consecutive days. One set of standards and samples was kept at room temperature while the other was stored in the refrigerator for the entire duration of the test period. Five replicate injections of the standard and samples were performed daily and monitored over five days. Daily mean peak areas at each concentration were calculated and %RSD calculated.
3 Quantitative Analysis of Ethanol in Locally Brewed Alcoholic Drinks
Following validation of the method, the collected 26 homemade alcoholic beverages were analysed.
4 Results and Discussions
4.1 Determination of Sample pH
The pH results obtained from the samples (Table 1) ranged from 2.31 to 4.8. These results agree with those reported by Mapitse et al. and Motlhanka et al. for the analysis of Khadi [44, 45]. The freshly prepared samples were found to have less acidic pH compared to those which were 1–2 days old. The fermentative process lowers the sample pH by increasing the level of lactic acid present. This in turn makes the samples safer for consumption because they are more resistant to microbial growth [54]. The results did not reveal any relationship between the sample pH and alcohol concentration.
4.2 Liquid–Liquid Extraction Results
The LLE studies were performed to determine the best solvent for the extraction of ethanol from the home-brewed alcoholic drinks. This was performed by first extracting 1 mL of each home-made alcoholic beverage with 1 mL of each of the following solvents: ethyl acetate, hexane, dichloromethane, and acetonitrile. The results for optimization of extraction (Fig. 4a, b), revealed that ethyl acetate with a sample to solvent ratio of 3:1 produced the highest recoveries (93.48%) when compared with the other three solvents (hexane, dichloromethane, and acetonitrile). Ethyl acetate is the most polar compared to hexane and dichloromethane and it is water immiscible solvent compared to acetonitrile and therefore the best extracting solvent for ethanol out of the four solvents.
4.3 Method Validation
4.3.1 Accuracy
The accuracy of the method was achieved by comparing the results of measured values with the true values of two known commercial alcohol samples of the Bombay (%v/v) and Cheteau Del Rei-Sweet (%v/v). The results are displayed in Table 2 below. The percentage (%) recovery results (Table 2) obtained at each concentration level ranged between 97.1 and 99.1%, indicating insignificant interference from the excipients. Several authors have also reported acceptable accuracy ranging from 90 to 110% [42, 55]. According to Kumar et al., acceptance criteria of accuracy range should at least be 80–120% of expected content [56]. Our results demonstrated that the method had sufficient capability for the accurate quantification of ethanol in home-brewed alcoholic samples.
4.3.2 Precision
4.3.2.1 Repeatability
Ten replicate injections were performed for each of the two ethanol standards comprising of 5% and 30% v/v ethanol concentration. The peak areas and %RSDs were calculated across the 10 injections for each standard. The results obtained for both standards (Table 3) showed % RSD values of 1.17 and 3.44 respectively, indicating excellent precision and that the method is fit for purpose.
4.3.3 Selectivity
The selectivity of the method was carried out by injecting a sample matrix spiked with possible interferents of home-brewed alcoholic beverages. The standard mixture was very well separated (Fig. 5). No components were found to interfere or elute at the same retention times of 5.674, 5.907, 6.134 7.853, 8.355 and 7.366 min for methanol, ethanol, acetone, 1-propanol, 1-butanol, and ethyl acetate, respectively. This confirms that the method is selective for this target analyte. The absence of interfering compounds with ethanol agrees with the recommendations of ICH and Scientific Group of Forensic Toxicology guidelines [53, 57].
4.3.4 Linearity and Analytical Range
Linearity and analytical range were assessed through the analysis of a series of standard solutions containing ethanol at nine concentration levels ranging from 2.5 to 60% v/v. The correlation coefficient (R2) of ethanol in ethyl acetate was found to be 0.996, confirming a linear relationship between the analyte and concentration. %RSD values for linearity concentrations ranged between 0.5 and 3.4% (Table 3). Based on the calibration results obtained, the developed method demonstrated linearity between ethanol concentrations of 2.5–60% v/v.
4.3.5 Limit of Detection (LOD) and Limit of Quantification (LOQ)
LOD and LOQ were determined using the Standard Deviation of the Y-intercept of regression lines as a standard deviation. Accordingly, the results obtained showed the values of 0.37 and 1.12 (µg/mL) for LOD and LOQ, respectively. The low LOD values obtained demonstrates that the method is sensitive [58].
4.3.6 Stability
The stability factor was assessed by monitoring the alcohol content of an in-house ethanol standard and the two samples of morula (A) and Setopoti (G) over five consecutive days. The content of both standards remained within the %RSD values of ≤ 5%, when stored for 5 days at room temperature and inside the refrigerator. Analyte stability values with %RSD values of ≤ 5% are considered stable [53, 59]. The alcohol content in the home brewed samples stored in the refrigerator remained stable for 5 days while those kept at room temperature were only stable for the first 2 days.
A summary of GCMS method validation results is presented in Table 3 below.
5 Quantitative Analysis of Ethanol in the 26 Samples of Locally Brewed Alcoholic Beverages
The validated method was subsequently used to determine the ethanol content of 26 locally brewed alcoholic beverages (Table 4), using the equation of the line; y = 607.76x−138 from the calibration curve. The results obtained revealed that the concentration of samples ranged between 2.56 and 36% v/v. The highest ethanol content observed was in Sekhokho (J) while the lowest was for Setopoti (G). The samples of Khadi collected from the Gantsi District showed an average ethanol content of 4.68% v/v. This was consistent with the Khadi results obtained by Mapitse et al. and Motlhanka et al. for samples collected in the Southern, Ngamiland and Central districts of Botswana [44, 45]. Alcohol content of khadi (K–V) and fermented morula (A, D, F), the only two types of homemade alcoholic beverages prepared from wild fruits, were in the same range of 3.31–6.11% v/v. The wide range of alcohol content for the same alcoholic beverage maybe due to different conditions, additives and methods of preparation which should be considered in the event of standardisation [45]. The alcohol content for the Chonchobina (X–Z) samples (10.51–11.21% v/v), made from a commercial sorghum malt powder and yeast as an additive, were higher than that of the Bojalwa jwa Setswana (H&I) (3.6–4.0% v/v). Even though both samples are made with sorghum malt, the higher alcohol content in the Chonchobina could be attributed to the addition of yeast, which in turn promotes excess fermentation.
In general, the ethanol content of the different Botswana homemade alcoholic beverages was consistent with those found in most commercial alcoholic beers, spirits, and wines (2.92–15.66% v/v) except for the Sekhokho sample (J), whose levels corresponded to that of distilled alcoholic beverages. The recipe for Sekhokho is a guarded secret amongst its brewers and remains a mystery to most Batswana.
One of the limitations of the study is that it does not include all the homemade alcoholic beverages of Botswana and that sampling was only limited to villages where the deaths were reported. In addition, due to lack of standardised methods of preparation, the ethanol content in the alcoholic beverage prepared by the same individual may differ from day to day. The quality of the samples is also likely to vary from one batch to another, due to the presence of additional ingredients on the brewers’ recipes.
6 Conclusion
Home-made alcohol-related deaths are a significant public health issue which is often overlooked. The major challenges with these types of beverages arise from batch-to-batch differences in product quality and safety due to variability of raw materials and non-standardised production methods. This method has proven to be inclusive of the different types of the available alcoholic beverages in Botswana, including Khadi. It is simple, rapid, cheap and does not require use of sophisticated instrumentation. It also requires use of minimal amount of solvent. Percentage analyte recoveries obtained were comparable with those reported previously [60, 61]. The presence of other components in the homemade alcoholic sample mixtures did not affect the identification and/or quantification of ethanol. There was no co-elution observed between ethanol and any of the likely excipients, indicating good selectivity. The lower LOD values obtained demonstrate that the method is highly sensitive. This method can be used for routine alcohol analysis in aqueous samples to provide reliable quantitative data combined with unquestionable confirmation of ethanol and many other volatile substances potentially present in home brewed fermented alcoholic beverages.
References
Rawat JM, Pandey S, Debbarma P, Rawat B (2021) Preparation of alcoholic beverages by tribal communities in the Indian Himalayan region: a review on traditional and ethnic consideration. Front Sustain Food Syst 5:240–259. https://doi.org/10.3389/fsufs.2021.672411
Anderson HE, Santos IC, Hildenbrand ZL, Schug KA (2019) A review of the analytical methods used for beer ingredient and finished product analysis and quality control. Anal Chim Acta 1085:1–20. https://doi.org/10.1016/j.aca.2019.07.061
Luczaj L, Jug-Dujakovic M, Dolina K, Vitasovic-Kosic I (2019) Plants in alcoholic beverages on the Croatian islands, with special reference to rakija travarica. J Ethnobiol Ethnomed 15(1):1–19. https://doi.org/10.1186/s13002-019-0332-1
Kazou M, Grafakou A, Tsakalidou E, Georgalaki M (2021) Zooming into the microbiota of home-made and industrial kefir produced in greece using classical microbiological and amplicon-based metagenomics analyses. Front Microbiol 12:64–80. https://doi.org/10.3389/fmicb.2021.621069
Manara LC (2021) Analysis of kefir information propagated in digital media. Int J Innov Educ Res 8(8):551–567. https://doi.org/10.3390/app12052459
Yılmaz I, Enver Dolar M, Ozpınar H (2019) Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: a randomized controlled trial. Turk J Gastroenterol 30(3):242–253. https://doi.org/10.5152/tjg.2018.18227
Holstege CP, Ferguson JD, Wolf CE, Baer AB, Poklis A (2004) Analysis of moonshine for contaminants. J Toxicol Clin Toxicol 42(5):597–601. https://doi.org/10.1081/clt-200026976
“Analysis of Moonshine for Contaminants # | Request PDF.” https://www.researchgate.net/publication/8253703_Analysis_of_Moonshine_for_Contaminants Accessed 12 Aug 2022
Rogerson CM (2019) African traditional beer: changing organization and spaces of South Africa’s sorghum beer industry. Afr Geogr Rev 38(3):253–267. https://doi.org/10.1080/19376812.2019.1589735
Debies-Carl JS (2019) Beyond the local: places, people, and brands in New England beer marketing. J Cult Geogr 36(1):78–110. https://doi.org/10.1080/08873631.2018.1511104
Pereira LM (2021) Follow the ‘Ting: sorghum in South Africa’. Food Cult Soc. https://doi.org/10.1080/15528014.2021.1984631
Deribe Y, Kassa E (2022) Value creation and sorghum-based products: what synergetic actions are needed?”. Cogent Food Agric 6(1):1–13. https://doi.org/10.1080/23311932.2020.1722352
Rabinovich L, Brutscher PB, De Vries H, Tiessen J, Clift J (2022) The affordability of alcoholic beverages in the European Union Understanding the link between alcohol affordability, consumption and harms. https://www.rand.org/pubs/technical_reports/TR689.html. Accessed 10 Aug 2022
“Prices of alcoholic beverages relative to prices of all consumer items.| Download Scientific Diagram.” https://www.researchgate.net/figure/Prices-of-alcoholic-beverages-relative-to-prices-of-all-consumer-items-December-1989-is_fig1_249995196. Accessed 12 Aug 2022
McCrady BS, Flanagan JC (2021) The role of the family in alcohol use disorder recovery for adults. Alcohol Res 41(1):12–24. https://doi.org/10.35946/arcr.v41.1.06
Social and cultural aspects of drinking—culture chemistry and consequences. http://www.sirc.org/publik/drinking6.html Accessed 12 Aug 2022
Alcohol consumption—Alcohol and society|Britannica. https://www.britannica.com/topic/alcohol-consumption/Alcohol-and-society. Accessed 12 Aug 2022
Irakiza G, Ugirinshuti V, Kamana O, Ongol MP (2021) Assessment of safety performance in banana alcoholic beverage processing factories in Rwanda. J Food Res 10(2):1–21. https://doi.org/10.5539/jfr.v10n2p1
Bayoï JR, Etoa FX (2021) Assessment of microbiological quality and safety during the processing of traditional beers made from sorghum in the ‘Mandara’ mountains of the Far-North Region of Cameroon. Eur J Biol Biotechnol 2(2):74–84. https://doi.org/10.24018/ejbio.2021.2.2.156
Kumar VR (2022) Forensic determination of adulterants in illicit liquor samples by using alcolyzer, densitometer and chemical methods. Mater Today Proc 11:221–229. https://doi.org/10.1016/j.matpr.2022.07.222
Aggarwal P, Kumar R, Pandey R, Singh S (2020) Identification of adulterants in liquor samples and their toxicity: a review. Seybold Rep 15:1768–1775. https://www.researchgate.net/publication/343336441_Identification_of_Adulterants_in_Liquor_Samples_and_Their_Toxicity_A_Review. Accessed 12 Aug 2022
Oduori DO, Kwoba E, Thomas L, Grace D, Mutua F (2022) Assessment of foodborne disease hazards in beverages consumed in Nigeria: a systematic literature review. Foodborne Pathog Dis 19(1):1–10. https://doi.org/10.1089/fpd.2021.0043
Doreen B (2017) Fatal methanol poisoning caused by drinking adulterated locally distilled alcohol: Wakiso District, Uganda. J Environ Public Health 11(2):20–29. https://doi.org/10.1155/2020/5816162
EXPLAINER: methanol in South Africa teen deaths often fatal. https://www.usnews.com/news/world/articles/2022-07-19/explainer-methanol-in-south-africa-teen-deaths-often-fatal. Accessed 21 July 2022
Drinkers died of methanol poisoning|Nation. https://nation.africa/kenya/news/drinkers-died-of-methanol-poisoning-982496. Accessed 8 June 2022
Hassanian-Moghaddam H (2015) Methanol mass poisoning in Iran: role of case finding in outbreak management. J Public Heal 37(2):354–359. https://doi.org/10.1093/pubmed/fdu038
Shale K, Mukamugema J, Lues R, Verma P (2014) Possible microbial and biochemical contaminants of an indigenous banana beer Urwagwa: a mini review. Afr J Food Sci 8(7):376–389. https://doi.org/10.5897/AJFS12.134
Destanoglu O, Ates I (2019) Determination and evaluation of methanol, ethanol and higher alcohols in legally and illegally produced alcoholic beverages. J Turk Chem Soc Sect A Chem 6(1):21–32. https://doi.org/10.1859/jotcsa.481384
Abdul RM, MutnuriL DPJ, Mohan DA (2012) Assessment of drinking water quality using ICP-MS and microbiological methods in the Bholakpur area, Hyderabad, India. Environ Monit Assess 184(3):1581–1592. https://doi.org/10.1007/s10661-011-2062-2
Two die after drinking traditional brew » TheVoiceBW. https://news.thevoicebw.com/two-die-after-drinking-traditional-brew/. Accessed 12 Aug 2022.
Three die after drinking traditional khadi brew » TheVoiceBW. https://news.thevoicebw.com/khadi-kills-three/. Accessed 8 June 2022
Botswana police service service-comparison of deaths/1000: Officers...|Download Scientific Diagram. https://www.researchgate.net/figure/Botswana-police-service-service-comparison-of-deaths-1000-Officers-and-sergeants_fig5_233363089. Accessed 8 June 2022
Botswana Police Forensic Laboraroty (2021) No Title, Gaborone
LaHood AJ, Kok SJ (2022) Ethanol toxicity. [Online]. https://www.ncbi.nlm.nih.gov/books/NBK557381/. Accessed 15 Aug 2022
Pitso JMN, Obot IS (2011) Botswana alcohol policy and the presidential levy controversy. Addiction 106(5):898–905. https://doi.org/10.1111/j.1360-0443.2011.03365.x
'Tho-tho-tho ‘Botswana traditional beer’—MUSIC AFRICA AWAKE. https://musicafricawakemedia.wordpress.com/2017/01/31/tho-tho-tho-botswana-traditional-beer/. Accessed 12 Aug 2022
Lachenmeier DW, Sarsh B, Rehm J (2009) The composition of alcohol products from markets in Lithuania and Hungary, and potential health consequences: a pilot study. Alcohol Alcohol 44(1):93–102. https://doi.org/10.1093/alcalc/agn095
Jones AW (2019) Alcohol, its analysis in blood and breath for forensic purposes, impairment effects, and acute toxicity. WIREs Forensic Sci 1(6):200–221. https://doi.org/10.1002/wfs2.1353
Gantumur MA (2019) Characterization of major volatile compounds in whey spirits produced by different distillation stages of fermented lactose-supplemented whey. J Dairy Sci. https://doi.org/10.3168/jds.2021-20748
Okaru AO, Lachenmeier DW (2021) Margin of exposure analyses and overall toxic effects of alcohol with special consideration of carcinogenicity. Nutrients 13(11):1–11. https://doi.org/10.3390/nu13113785
Gschaedler A, Iñiguez-Muñoz LE, Flores-Flores NY, Kirchmayr M, Arellano-Plaza M (2021) Use of non-Saccharomyces yeasts in cider fermentation: importance of the nutrients addition to obtain an efficient fermentation. Int J Food Microbiol 347:1–23. https://doi.org/10.1002/dta.3241
Frei P, Frauchiger S, Scheurer E, Mercer-Chalmers-Bender K (2022) Quantitative determination of five cannabinoids in blood and urine by gas chromatography tandem mass spectrometry applying automated on-line solid phase extraction. Drug Test Anal 2(1):20–22. https://doi.org/10.1002/dta.3241
Kovac M (2021) Mycotoxins, pesticide residues, and heavy metals analysis of croatian cereals. Microorganisms 9(2):1–21. https://doi.org/10.3390/microorganisms9020216
Mapitse R, Okatch H, Moshoeshoe E (2014) Analysis of volatile compounds in khadi (an unrecorded alcohol beverage) from Botswana by gas chromatography-flame ionization detection (GC-FID). South Afr J Chem 67(1):184–188. http://www.ajol.info/index.php/sajc/article/view/122492
Motlhanka K, Lebani K, Boekhout T, Zhou N (2021) Fermentative microbes of khadi, a traditional alcoholic beverage of Botswana. Fermentation 6(51):1–20. https://doi.org/10.3390/fermentation6020051
Bettenhausen HM (2018) Influence of malt source on beer chemistry, flavor, and flavor stability. Food Res Int 113:487–504. https://doi.org/10.1016/j.foodres.2018.07.024
Yang YN, Liang M, Yang Y, Zheng FP, Wang XP, Yu YN (2020) Optimization of a headspace solid-phase microextraction method for the gas chromatography-mass spectrometry analysis aroma compounds of Litsea mollis Hemsl. immature fruit. Food Sci Technol 40(4):786–793. https://doi.org/10.1590/fst.20319
de Galvão MS (2020) Method optimization study on isolation of volatile compounds by headspace solid-phase microextraction (HS-SPME) from custard apple (Annona squamosa L.). Pulp J Anal Sci Methods Instrum 10(3):59–77. https://doi.org/10.4236/jasmi.2020.103005
Royal Society of Chemistry. Advancing analytical chemistry in Africa—inspired by a skip in Liverpool! Jan. 2018, [Online]. https://www.rsc.org/news-events/features/2018/jan/lcms-workshop/. Accessed 12 Aug 2022
Bell L (2018) Challenges and opportunities for biological mass spectrometry core facilities in the developing world. J Biomol Tech 29(1):4–8. https://doi.org/10.7171/jbt.18-2901-003
Chun HJ, Poklis JL, Poklis A, Wolf CE (2018) Determination of ethanol in blood using Liquid-liquid Extraction gas chromatography withflameionization detector (LLE-GC-FID): validation ofa method. J Anal Toxicol 40(8):653–658. https://doi.org/10.1080/23312009.2020.1760187
Schematic representation of LLE. Download scientific diagram. https://www.researchgate.net/figure/Schematic-representation-of-LLE_fig1_350633245. Accessed 15 Aug 2022
ICH Official web site: ICH. https://www.ich.org/. Accessed 23 June 2022
Why is it important to lower the PH of fermented foods?|eHow. https://www.ehow.com/about_5467277_important-lower-ph-fermented-foods.html. Accessed 15 Aug 2022
Lee SM, Pyeon YK, Chung MS, Kim YS (2022) Determination of methanol and fusel oils in various types of wines distributed in Korea. Food Sci Biotechnol 31(2):203–209. https://doi.org/10.1007/s10068-021-01026-3
Kumar A, Kishore L, Kaur N, Nair A (2020) Method development and validation: Skills and tricks. Chron Young Sci. https://doi.org/10.4103/2229-5186.94303
Scientific Working Groups in the Forensic Sciences|National Institute of Justice. https://nij.ojp.gov/topics/articles/scientific-working-groups-forensic-sciences. Accessed 12 Aug 2022
Guo WY (2021) Multienzyme-targeted fluorescent probe as a biosensing platform for broad detection of pesticide residues. Anal Chem 93(18):7079–7085. https://doi.org/10.1021/acs.analchem.1c00553
FDA. Guidances (drugs) https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs. Accessed 12 Aug 2022
Sarraf M, Beig-babaei A, Naji-Tabasi S (2020) Application of QuEChERS method for extraction of functional compounds. SN Appl Sci 2(11):1–6. https://doi.org/10.1007/s42452-020-03639-5
Assaye M, Tamirat B, Fekadu B (2021) Ethanol concentration and calorific value of some local distilled Ethiopian alcohol (Areki): an energy potential assessment. Cogent Eng J 8(1)2021. https://doi.org/10.1080/23311916.2021.1979444. http://www.editorialmanager.com/cogenteng. Accessed 3 July 2022
Acknowledgements
The authors would like to thank the Botswana International University of Science and Technology (BIUST) for providing funds and facilities for this project, Botswana Police service for their collaborative efforts and the Ministry of Health for granting us permission to conduct the study.
Funding
This research work was financially supported by the Botswana International University of Science and Technology (BIUST), Research Initiation Grant Number BIUST/R00090/2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors report no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsenang, M., Pheko, T., Mokgadi, J. et al. A Validated Liquid–Liquid Extraction Method for the Quantitative Analysis of Ethanol in the Different Types of Home-Brewed Alcoholic Beverages of Botswana Using Gas Chromatography Flame Ionization Detector. Chemistry Africa 6, 417–427 (2023). https://doi.org/10.1007/s42250-022-00520-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00520-3