Abstract
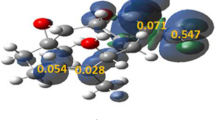
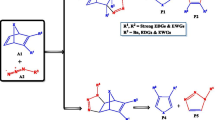
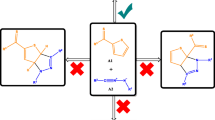
The intramolecular [3+2] cycloaddition (32CA) reactions of azido alkynes leading to spirocyclic, tricyclic, and bicyclic triazolooxazines has been studied within the molecular electron density theory (MEDT) at the MPWB1K/6-311G(d,p) level. The electron localization function (ELF) characterizes the azido alkynes as zwitterionic species. Analysis of the conceptual DFT indices allows classifying the azide moiety as the electrophilic counterpart and the alkyne as the nucleophilic one. These 32CA reactions are under kinetic control with the activation free energies of 23.4–26.7 kcal mol−1. Along the reaction path, the pseudoradical centre is created initially at C4, consistent with the Parr function analysis; however, the sequence of bond formation is controlled by the energetically feasible formation of the six-membered oxazine ring. The intermolecular interactions at the transition states were characterized from the quantum theory of atoms in molecules (QTAIM) study and the non-covalent interaction (NCI) gradient isosurfaces.












Similar content being viewed by others
Data availability
All datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Huisgen R (1984) In: Padwa A (ed) 1,3-Dipolar cycloaddition—introduction, survey, mechanism. Wiley, New York
Huisgen R, Knorr R, Moebius L, Szeimies G (1965) 1.3-Dipolare cycloadditionen, XXIII. Einige Beobachtungen zur Addition organischer Azide an CC-Dreifachbindungen. Chem Ber 98:4014–4021. https://doi.org/10.1002/cber.19650981228
Krivopalov VP, Shkurko OP (2005) 1,2,3-Triazole and its derivatives. Development of methods for the formation of the triazole ring. Russian Chem Rev 74:339–379. https://doi.org/10.1070/RC2005v074n04ABEH000893
Arslan M, Acik G, Tasdelen MA (2019) The emerging applications of click chemistry reactions in the modification of industrial polymers. Polymer Chemistry 10:3806–3821. https://doi.org/10.1039/C9PY00510B
Hein CD, Liu XM, Wang D (2008) Click chemistry: a powerful tool for pharmaceutical sciences. Pharma Res 25:2216–2230. https://doi.org/10.1007/s11095-008-9616-1
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) A stepwise Huisgen cycloaddition process: copper(l)-catalyzed regioselective "Ligation" of azides and terminal alkynes. Angew Chem Int Ed 41:2596–2599. https://doi.org/10.1002/1521-3773(20020715)41:14%3C2596::aid-anie2596%3E3.0.co;2-4
Tornøe CW, Meldal M (2001) In: Lebl M, Houghten RA (eds) The wave of the future. American Peptide Society, San Diego
Tornøe CW, Christensen C, Meldal M (2002) Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem 67:3057–3064. https://doi.org/10.1021/jo011148j
Nebra N, García-Álvarez J (2020) Recent progress of Cu-catalyzed azide-alkyne cycloaddition reactions (CuAAC) in sustainable solvents: glycerol, deep eutectic solvents, and aqueous media. Molecules 25:2015. https://doi.org/10.3390/molecules25092015
Boren BC, Narayan S, Rasmussen LK, Zhang L, Zhao H, Lin Z, Jia G, Fokin VV (2008) Ruthenium-catalyzed azide−alkyne cycloaddition: scope and mechanism. J Am Chem Soc 130:8923–8930. https://doi.org/10.1021/ja0749993
Li R, Jansen DJ, Dutta A (2009) Intramolecular azide-alkyne[3 + 2] cycloaddition: versatile route to new heterocyclic structural scaffolds. Org Biomol Chem 7:1921–1930. https://doi.org/10.1039/B818962E
Majumdar KC, Ray K, Ganai S (2010) Intramolecular Azide-Alkyne [3+2] Cycloaddition: a versatile route for the synthesis of 1,2,3-triazole fused dibenzo[1,5]diazocine derivatives. Synthesis 12:2101–2105. https://doi.org/10.1055/s-0029-1218763
Mazur MO, Zhelavskyi OS, Zviagin EM, Shishkina SV, Musatov VI, Kolosov MA, Shvets EH, Andryushchenko AY, Chebanov VA (2021) Effective microwave-assisted approach to 1,2,3-triazolobenzodiazepinones via tandem Ugi reaction/catalyst-free intramolecular azide–alkyne cycloaddition. Beilstein J Org Chem 17:678–687. https://doi.org/10.3762/bjoc.17.57
Cobb AJA, Dell’Isola A, Abdulsattar BO, McLachlan MMW, Neuman BW, Müller C, Shankland K, Al-Mulla HMN, Binks AWD, Elvidge W (2018) Synthesis and antiviral activity of novel spirocyclic nucleosides. New J Chem 42:18363–18380. https://doi.org/10.1039/C8NJ02777C
Domingo LR (2016) Molecular electron density theory: a modern view of reactivity in organic chemistry. Molecules 21:1319. https://doi.org/10.3390/molecules21101319
Ríos-Gutiérrez M, Domingo LR (2019) Unravelling the mysteries of the [3+2] cycloaddition reactions. Eur Jour Org Chem 2019:267–282. https://doi.org/10.1002/ejoc.201800916
Domingo LR, Acharjee N (2020) In Frontiers in computational chemistry, Ul-Haq Z, Wilson AK (ed(s)) Bentham and Science, Singapore, p. 174-227. https://doi.org/10.2174/9789811457791120050007
Domingo LR, Acharjee N (2020) Unravelling the strain-promoted [3+2] cycloaddition reactions of phenyl azide with cycloalkynes from the molecular electron density theory perspective. New J Chem 44:13633–13643. https://doi.org/10.1039/D0NJ02711A
Domingo LR, Acharjee N (2020) Unveiling the high reactivity of strained dibenzocyclooctyne in [3+2] cycloaddition reactions with diazoalkanes through the molecular electron density theory. J Phys Org Chem 33:e4100. https://doi.org/10.1002/poc.4100
Domingo LR, Acharjee N (2021) Unveiling the substituent effects in the stereochemistry of [3+2] cycloaddition reactions of aryl- and alkyldiazomethylphosphonates with norbornadiene within a MEDT perspective. ChemistrySelect 6:10722–10733. https://doi.org/10.1002/slct.202102942
Domingo LR, Acharjee N (2018) [3+2] Cycloaddition reaction of C-phenyl-N-methyl nitrone to acyclic-olefin-bearing electron-donating substituent: a molecular electron density theory study. ChemistrySelect 3:8373–8380. https://doi.org/10.1002/slct.201801528
Domingo LR, Ríos-Gutiérrez M, Acharjee N (2019) A molecular electron density theory study of the chemoselectivity, regioselectivity, and diastereofacial selectivity in the synthesis of an anticancer spiroisoxazoline derived from α-santonin. Molecules 24:832. https://doi.org/10.3390/molecules24050832
Domingo LR, Acharjee N (2021) Unveiling the chemo- and regioselectivity of the [3+2] cycloaddition reaction between 4-chlorobenzonitrile oxide and β-aminocinnamonitrile with a MEDT perspective. ChemistrySelect 6:4521–4532. https://doi.org/10.1002/slct.202100978
Domingo LR, Ríos-Gutiérrez M, Pérez P (2018) A molecular electron density theory study of the reactivity and selectivities in [3 + 2] cycloaddition reactions of C, N-dialkyl nitrones with ethylene derivatives. J Org Chem 83:2182–2197. https://doi.org/10.1021/acs.joc.7b03093
Acharjee N, Salim HAM, Chakraborty M, Rao MP, Ganesh M (2021) Unveiling the high regioselectivity and stereoselectivity within the synthesis of spirooxindolenitropyrrolidine: a molecular electron density theory perspective. J Phys Org Chem 34:e4189. https://doi.org/10.1002/poc.4189
Domingo LR, M. Ríos-Gutiérrez M, Pérez P (2018) A molecular electron density theory study of the role of the copper metalation of azomethine ylides in [3 + 2] cycloaddition reactions. J Org Chem 83:10959–10973. https://doi.org/10.1021/acs.joc.8b01605
Ayouchia HBEl, Lahoucine B, Anane H, Ríos-Gutiérrez M, Domingo LR, Stiriba SE (2018) Experimental and theoretical MEDT study of the thermal [3+2] cycloaddition reactions of aryl azides with alkyne derivatives. ChemistrySelect 3:1215–1233. https://doi.org/10.1002/slct.201702588
Ayouchia HBEl, Lahoucine B, Anane H, Domingo LR, Stiriba SE (2018) Understanding the mechanism and regioselectivity of the copper(I) catalyzed [3 + 2] cycloaddition reaction between azide and alkyne: a systematic DFT study. RSC Adv 8:7670–7678. https://doi.org/10.1039/C7RA10653J
Domingo LR, Acharjee N (2020) A molecular electron density theory study of the grignard reagent mediated regioselective direct synthesis of 1,5-disubstituted-1,2,3-triazoles. J Phys Org Chem 33:e4062. https://doi.org/10.1002/poc.4062
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 21:748. https://doi.org/10.3390/molecules21060748
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1874. https://doi.org/10.1021/cr990029p
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92:5397–5403. https://doi.org/10.1063/1.458517
Silvi B, Savin A (1994) Classification of chemical bonds based on topological analysis of electron localization functions. Nature 371:683–686. https://doi.org/10.1038/371683a0
Bader RFW (1994) Atoms in molecules: a quantum theory. Clarendon Press, USA
Bader RFW, Essén H (1984) The characterization of atomic interactions. J Chem Phys 80:1943–1960. https://doi.org/10.1063/1.446956
García JC, Johnson ER, Keinan S, Chaudret R, Piquemal JP, Beratan DN, Yang W (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632. https://doi.org/10.1021/ct100641a
Schlegel HB (1982) Optimization of equilibrium geometries and transition structures. J Comput Chem 3:214–218. https://doi.org/10.1002/jcc.540030212
Zhao Y, Truhlar DG (2004) Hybrid meta density functional theory methods for thermochemistry, thermochemical kinetics, and noncovalent interactions: the MPW1B95 and MPWB1K models and comparative assessments for hydrogen bonding and van der Waals interactions. J Phys Chem A 108:6908–6918. https://doi.org/10.1021/jp048147q
Hehre WJ, Radom L, Schleyer PVR, Pople JA (1996) Ab initio molecular orbital theory. Wiley, New York, USA
Fukui K (1970) Formulation of the reaction coordinate. J Phys Chem 74:4161–4163. https://doi.org/10.1021/j100717a029
González C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527. https://doi.org/10.1021/j100377a021
González C, Schlegel HB (1991) Improved algorithms for reaction path following: Higher-order implicit algorithms. Chem Phys 95:5853–5860. https://doi.org/10.1063/1.461606
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, Oxford 4:70–86
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516. https://doi.org/10.1021/ja00364a005
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 58:4417–4423. https://doi.org/10.1016/S0040-4020(02)00410-6
Domingo LR, Pérez P (2011) The nucleophilicity N index in organic chemistry. Org. Biomol Chem 9:7168–7175. https://doi.org/10.1039/C1OB05856H
Domingo LR, Pérez P, Sáez JA (2013) Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv 3:1486–1494. https://doi.org/10.1039/C2RA22886F
Tomasi J, Persico M (1994) Molecular Interactions in Solution: An Overview of Methods Based on Continuous Distributions of the Solvent. Chem Rev 94:2027–2094. https://doi.org/10.1021/cr00031a013
Cances E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107:3032–3041. https://doi.org/10.1063/1.474659
Barone V, Cossi M, Tomasi J (1998) Geometry optimization of molecular structures in solution by the polarizable continuum model. J Comput Chem 19:404–417. https://doi.org/10.1002/(SICI)1096-987X(199803)19:4/404::AID-JCC3/3.0.CO;2-W
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Humphrey W, Dalke A, Schulten K (1996) VMD: Visual molecular dynamics. J Molec Graphics 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Petersson G, Nakatsuji H (2016) Gaussian 16.Revision A 3
Thom R (1972) StabilitéStructurelle et Morphogénèse. Intereditions, Paris
Krokidis X, Noury S, Silvi B (1997) Characterization of elementary chemical processes by Catastrophe Theory. J Phys Chem A 101:7277–7282. https://doi.org/10.1021/jp9711508
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by Nivedita Acharjee, Haydar A Mohammad-Salim, and Mrinmoy Chakraborty. The first draft of the manuscript was written by Nivedita Acharjee, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Acharjee, N., Mohammad-Salim, H.A. & Chakraborty, M. Unveiling the synthesis of spirocyclic, tricyclic, and bicyclic triazolooxazines from intramolecular [3 + 2] azide-alkyne cycloadditions with a molecular electron density theory perspective. Struct Chem 33, 555–570 (2022). https://doi.org/10.1007/s11224-021-01870-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01870-3