Abstract
In this study, starch-graft-poly (methyl methacrylate) (starch-g-PMMA) composites doped with different amounts (5, 10, and 15 wt%) of cadmium sulfide (CdS) particles were fabricated for electrical measurements using in-situ polymerization technique. The structural characterization of the samples was studied. The dielectric and conductivity properties of the composites were investigated using impedance analyzer depending on the frequency (100 Hz–20 kHz) at room temperature. Spectroscopy revealed that the dielectric constant (ε′), dielectric loss (ε′′), and real (Z) components of impedance are found to decrease with increasing frequency for both starch-g-PMMA and starch-g-PMMA (5, 10, and 15 wt%) composites. On the contrary, the AC conductivity (σac) of samples increased with increasing frequency. In addition, ε′ and ε′′ values of starch-g-PMMA were lower than composites that added CdS. When the CdS content increased from 0 to 15%, ε′ increased from 5.62 to 15.10 at 100 Hz. AC conductivity was improved after adding the CdS particles. The maximum conductivity value for starch-g-PMMA15%CdS is found to be 3.07 × 10–7 at 100 Hz. When the dielectric properties of composites are evaluated, it is concluded that it is an exciting material for electronic applications in technology.
Similar content being viewed by others
Introduction
Research on polymers is becoming a major field due to their simple preparation and widespread use. Blends prepared with compatible polymers become very important because they are cheap, simple to prepare and can be used in different applications [1]. The processability and affordability of polymers are the dominant factors in their use in daily life [2].
Starch is abundant and biodegradable polymer. It has been used as many industrial products. Among the many natural raw resources, starch is considered one of the most important and promising candidates because it is abundant, renewable, low-cost, and completely biodegradable. Starch has limited functionality for use in various applications due to its poor physical properties, high hydrophilicity, high recrystallization behavior, and low decomposition temperature. For this purpose, chemical and physical modification processes are carried out. The modification provides a viable way to combine the advantages of both natural polysaccharides and synthetic polymers. Chemical modification and methods such as acetylation and acid hydrolysis have been applied. Physical modifications are conditions such as electrical conductivity, ultrasound, heat-humidity, and annealing [3,4,5,6]. Starch has a strong intermolecular hydrogen bond structure consisting of amylopectin and amylose in a supramolecular and semi-granular structure. Grafting is a modification method to change the properties of polymers and thus enables the polymers to be bonded to each other [7]. Natural polymers are frequently provided as flocculants in surface waters and industrial wastewater treatment. Grafting is a method used to change the chemical and physical properties of natural and synthetic polymers. Grafting has been used as both recovery applications and flocculant agents. Chemical modification of starch through vinyl graft copolymerization establishes a potential way to improve starch properties and thus expand its range of uses. Grafting is generally regarded to result from the spread of radical sites created on the polymer substrate [8]. PMMA is a cheaper alternative according to many compounds in applications such as tensile strength, flexural strength, transparency, polishability, and UV tolerance. Thus, the PMMA is selected.
Starch's rich content, abundance, edibility, biodegradability, and electrical properties have engaged attention of researchers [9, 10]. Starch has a strong intermolecular hydrogen bond structure consisting of amylopectin (α-(1–4)-bonded D-glucose backbone and about 5% α-(1–6)-bonded branches) and amylose (α-(1–4)-bonded D-glucose units) in a supramolecular and semi-granular structure. To obtain starch as a conductive material, heat treatment in an ionic solvent or ionically conductive materials can be used [11, 12, 65, 66].
Electrical conductivity is expressed as the increase in water and moisture content of a sample during the heating process. Electrical conductivity is a function of time and temperature [13]. As a result of their significant electrical conductivity, polymers are used in many electronic fields [14,15,16].
Gelatinization is the disruption of molecular order as a result of heating starch in an aqueous environment. In a similar situation, it was noticed that the conductivity increased linearly with the increase in temperature and also increased at the maximum level with the increase in water content [17]. Bauner and Knor [18] reported that the electrical conductivity and degree of gelatinization of wheat starch and tapioca starch suspensions increased significantly after 530 MPa, and the electrical conductivity and degree of gelatinization increased linearly with increasing pressure. In another study, Wang et al. [19] reported that, as a result of ohmic heating, electrical conductivity increased with temperature, but the gelation degree decreased. Chaiwanichsiri et al. [20] studied the electrical conductivity of 12 different starch sources and reported that their electrical conductivity increased with temperature.
El-Gamal studied the mechanical, optical, and electrical properties by adding PMMA, a compatible polymer, to polystyrene (PS), and the results reported that PMMA both reduced the cost and increased its optical and electrical properties [21]. Poly methyl methacrylate (PMMA), polyvinyl alcohol (PVA), polyaniline (PANI), and polyindole (PIN) are good polymeric materials with their durability, easy synthesis, cheapness, reprocessability, flexibility, resistance to corrosion, and excellent physical and optical properties. Among these, PMMA is widely used in lenses, light pipes, skylights, inorganic glass industry, and light-emitting devices due to its lightness, corrosion resistance, transparency, and electrical properties [21,22,23,24,25,26]. In a study, it was reported that PMMA improved both the mechanical and optical properties of polyaniline (PANI) film [27, 62,63,64].
By adding inorganic materials to the polymer matrix, it provides the opportunity to use optoelectronic or microelectronic devices as a result of physical and chemical interactions [28]. Among II-VI semiconductor nanomaterials, crystalline cadmium sulfide has attracted attention due to its electrical properties [29, 30]. The aim of this study is to examine the electrical properties of the composite with CdS doped on starch-g-PMMA polymer after being characterized by X-ray diffraction (X-ray), scanning electron microscopy-energy-dispersive spectroscopy (SEM–EDS), and Fourier transform infrared spectroscopy (FTIR). The effects of CdS in the polymer matrix on dielectric constant, dielectric loss, and conductivity at different frequencies were examined. It is to prove that dielectric properties increase as the amount of additive increases. CdS is a semiconductor substance. It was aimed to emphasize how the polymer, which has insulating properties, affects the conductivity behavior. In addition, the electrical applicability of this biodegradable and environmentally friendly polymer using CdS has not been studied before.
Materials and methods
Starch (Mw: 162 g/mol) and methyl methacrylate (MMA) monomer were purchased from Acros (Mw: 100.12 g/mol). Ethanol, azobisisobutyronitrile (AIBN), and dimethyl formamide were obtained from Sigma–Aldrich. Acetone was supplied by Merck firm. Semiconductor cadmium sulfide (Mw: 112.41 g/mol) was provided from Boston company. All chemicals were used without any purification process.
Preparation of cadmium sulfur-added starch-graft-poly (methyl methacrylate) composites
First of all, as the first step, 2 g of starch was dissolved at 40 °C for 30 min. Then, 4-g MMA and AIBN dissolved in 1-ml acetone at 60 °C for 24 h were added to the mixture. Ethanol was added to the mixture, precipitated, and dried, resulting in starch-g-PMMA. In the second stage, 0.5 g of starch-g-PMMA and CdS at different ratio were added and dissolved in dimethyl formamide for 1 h. Then the solutions were precipitated and dried at 50 °C. The preparation of polymer composite is shown in Scheme 1.
Structural characterizations
Fourier transform infrared spectroscopy (FTIR) spectra were utilized to identify chemical groups. FTIR spectra of the samples were carried out with a Jasco 6700 instrument in the 400–4000 wavelength range and 32 scans. X-ray diffraction spectroscopy (Rigaku MiniFlex X-ray) was accomplished with Cu-Kα radiation (λ = 1.5406 Å) to determine the crystal structure of the sample. XRD spectra were scanned from 10° to 80°. The topographic features of the sample surface were imaged with scanning electron microscopy (SEM). Then, the sample was studied with an energy-dispersive spectroscopy (EDS) analyzer to explain the composition of the elements. Hitachi S-3500 SEM–EDS instrument was used to perform these analyses.
Dielectric properties of the starch-g-PMMA composites
The dielectric properties of the starch-g-PMMA and its composites were studied as a function of frequency at room temperature using a Novocontrol Technologies (Alpha‐AN) impedance analyzer specifications 100 Hz to 20 kHz. The composites whose dielectric and conductivity properties were examined are in the solid phase. Pellets were prepared by taking 0.2 g of these composites under 5 tons of pressure. Disk-shaped specimens had a diameter of about 50 mm and a thickness of about 5 mm. The dielectric constant (ε′), dielectric loss (ε′′), and AC conductivities (σac) were calculated using Eqs. (1), (2), and (3) [50, 51]. The real (Z′) components of impedance were measured by impedance analyzer.
where C is the capacitance of the composites measured from 100 to 20 kHz, d is the thickness, A is the area, ε0 is the permittivity of free space permittivity (8.854 × 10−12 F/m), DF is the dissipation factor, and G is the conductance. The impedance was measured by impedance analyzer.
Results and discussion
FTIR spectra
Figure 1 depicts FTIR spectra of starch-g-PMMA and doped CdS in polymer matrix. The presence of functional groups in the polymer matrix is verified by FTIR spectroscopy. The wide and broad band between 3100 and 3600 cm−1 ascribes to the absorption peak of starch due to OH stretching. The bands at 2997 and 2952 cm−1 are C–H stretching vibrations. The band at 1723 cm−1 belongs to the grafted carbonyl group (C=O) stretching in the structure of PMMA [7]. The peak at 1435 cm−1 can be referred to the asymmetric stretching of PMMA responsible for CH3. The stretchings at 1239 and 838 cm−1 are responsible for the C–O and C–O–C stretching, respectively. The characteristic peak at 1386 cm−1 can be mentioned as the OCH3 deformation stress of PMMA [8, 21]. The 407 cm−1 stretching is the CdS absorption band [31]. The vibrational stretching between 400 and 700 cm−1 represents the metal sulfide region [32]. With the addition of CdS, a small absorption peak was observed at 3437 cm−1, and as a result, the intensity and width of the peak decreased.
XRD
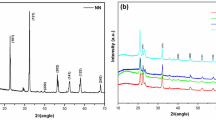
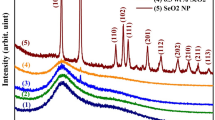
Figure 2 presents the XRD patterns of samples with and without CdS addition to the polymer matrix. 14.81° and 21.80° pertain to the XRD pattern of starch in amorphous structure. In Fig. 2b, sharp peaks are seen in many different crystalline structures. In particular, the diffractions corresponding to 26.22° (1, 1, 0), 44.55° (2, 2, 0), and 52.37° (3, 1, 1) indicate the presence of CdS and that it represents the hexagonal structure. Peaks with a wide diffraction structure, particle size decreases when the peak width increases, and this provides information about the crystal structure. Similar situations were expressed in previous studies [29, 31,32,33,34]. As a result of CdS loading, a distinct pattern was observed compared to the pattern of starch-PMMA. Starch is an amorphous polymer. In starch-g-PMMA, the XRD pattern of the peaks is crystalline. An increase in crystalline structure is observed with the addition of CdS [61].
SEM–EDS
The surface morphology and EDS of samples doped with different amounts of CdS and the composition of the elements in the polymer are given in Fig. 3. As can be seen from the images, agglomeration is observed in all proportions of CdS. Agglomeration of CdS constitutes larger masses with increasing concentration, as seen in Fig. 3b–d. The agglomerations appear porous and needle-shaped. Previous work stated that agglomerations occurred when CdS was included in the PVA matrix [31]. The surface of starch-g-poly(methacrylate) without CdS is rough and has crystalline flakes. With the addition of CdS, changes occurred on the sample surface. If the SEM images of the polymer composite are examined, the surface roughness decreases when CdS is incorporated to starch-g-poly(methacrylate). With increasing CdS ratio, crystalline flakes in the starch-g-poly(methacrylate) structure disappeared. This explains the distribution of CdS within the polymer network. The distribution of atoms in the polymer and CdS-containing samples is mapped in Fig. 4. EDS results are presented in Table 1. Starch-g-PMMA sample is 54.7% carbon (C) and 45.3% oxygen. The cadmium metal contents of starch-g-PMMA containing 5, 10, and 15% CdS are 3.3, 6.2, and 13.8%, respectively. According to the EDS results, it was observed that the CdS in the polymer matrix increased in elemental percentage.
Dielectric constant
The visual regarding the application area of the CdS-doped polymer matrix is illustrated in Scheme 2. A material is classified as a "dielectric" if it has the ability to store energy when an external electric field is applied. The dielectric constant (έ) shows how much energy is stored in the outer electric region and how much energy is lost in the material under the influence of a field [35]. Based on the physics theory, it can be said that the main factor affecting the έ of the material is polarization. έ is affected by factors such as atomic polarization, electron polarization, dipole polarization, and interfacial polarization [36, 37]. Compared to others, dipole polarization occurs in the low frequency and takes longer. Another important factor affecting the έ of a material is its polarity. The higher the polarity of the substance, the higher its dielectric constant. This is why polymers containing many polar groups are chosen in polymer composites when high έ is desired [38]. In this study, the effect of CdS on the έ of starch-g-PMMA was examined depending on frequency at room temperature and is shown in Fig. 5. As seen in the figure, for each sample, the έ decreased with increasing frequency. This may be due to the limitation of dipole mobility at high frequencies. Additionally, this phenomenon is attributed to the slower response of the dipoles due to their large moment of inertia. So they are not able to follow the rapidly varying electric field, and their contribution to polarization ceases. Damping of these dipoles accounts for the decrease in dielectric constant at higher frequencies. With an increase in frequency, the dielectric constant further decreases and reaches a constant value of about 500 Hz. As seen in Fig. 5, έ of composites is higher than pure polymer, and it increased with increasing filler content. At a frequency of 100 Hz, with a CdS content of 15 wt%, the έ value of the polymer increase from 5.62 to 15.10. The increase in έ with increase in dopant amount is a result of the interfacial polarization effect between the starch-g-PMMA and CdS particles [39,40,41]. This result is compatible with many studies. In a study conducted by Mondal et al., CdS nanocomposites have been grown in a polymer (polyvinyl alcohol). The nanocomposites show an enormous enhancement of dielectric constant in polyvinyl alcohol matrix over a frequency range of 40 Hz–10 MHz [52]. A distinct interphase region surrounds the conducting phase embedded in the polymer. This interphase region is formed due to strong interactions between functional groups of the polymer and the surface of the nanoclusters through covalent or hydrogen bonds. In a study presented by Kalandaragh, dielectric properties of pure PVA and CdS-PVA nanocomposite are characterized in temperature range of 298 to 498 K and frequency range from 200 Hz to 1 MHz. The dielectric constant decreases with increase in frequency at all temperatures for both pure PVA and CdS-PVA nanocomposite. The values of dielectric constant in CdS-PVA nanocomposite are large, and this is connected with small size of CdS particles [53]. Bhunia et al. prepared free-standing flexible composite films of nanocrystalline cadmium sulfide-impregnated poly(vinylidene fluoride) (nano-CdS/PVDF) using a sol–gel technique. The effect of CdS loading, in the PVDF host matrix, on the dielectric properties was studied. An increase in dielectric constant (more than 10 times) was observed in the films when poled under an electric field [54]. έ values are summarized in Table 2.
Dielectric loss
Dielectric loss (εʺ), which is an important parameter in material selection, is a parameter that shows how much of the energy given to the material is consumed as heat within the material. [42, 43]. Figure 6 shows the decrease in εʺ with increasing frequency of composites prepared with different content of CdS and pure polymer at room temperature. It is clearly seen that εʺ values decrease with increasing frequency and remains constant at high frequencies for all samples. The higher value of εʺ at low frequency is due to free charge movement within the polymer [44]. In addition, the εʺ increases with the increase in filler amount, which is attributed to interfacial polarization mechanisms in a heterogeneous system. The εʺ values for starch-g-PMMA, starch-g-PMMA5%CdS, starch-g-PMMA10%CdS, and starch-g-PMMA15%CdS composites were found to be 1.65 × 10–1, 2.79 × 10–1, 3.32 × 10–1, 1.05 at 100-Hz frequency, respectively. Due to their good conductivity, the conductive fillers easily form conductive paths by contacting each other, which causes current leakage and increases the dielectric loss of the samples. Khurana and Jaggi reported that dielectric constant, dielectric loss, and AC conductivity of CdS nanostructures were carried out in frequency range 1 kHz to 5 MHz at different temperatures. Dielectric loss decreased as frequencies increased. This low value of dielectric loss at high frequency relates to the purity of the nanoparticles, having lesser defects and good optical quality which is further useful in enhanced optical devices [55]. It is observed that the dielectric constant and dielectric loss have low values at high-frequency region and are independent of temperature. This behavior of dielectric at high frequency confirms the sample owns the optical quality with lesser defects. The nanocomposite samples of polyaniline (PANI) and lead sulfide nanoparticles (PbSNPs) were prepared by Althubiti et al. The induced changes in the dielectric properties and structural characteristics of the PANI/PbS samples were determined. They reported that the dielectric constant, dielectric loss, and the electrical conductivity were improved by enhancing the fluence to 15 × 1016 ions.cm−2 [56]. Althubiti et al. synthesized the flexible polymeric composite films composed of polyaniline (PANI), silver nanoparticles (AgNPs), and methylcellulose (MC) using solution cast preparation method. They reported that, after bombarding the original film with an ion beam, other optical parameters, such as dielectric loss and optical conductivity, were modified, while the relaxation time was reduced [57]. εʺ values are summarized in Table 2.
Conductivity
Figure 7 shows the change of electrical conductivities of pure starch-g-PMMA and its composites which were prepared with different content of CdS depending on frequency at room temperature. It is clear that the σac increases linearly with increasing the frequency. The conductivity value increased from 2.57 × 10−10 S/cm to 2.66 × 10−9 S/cm and 4.80 × 10−8 S/cm for starch-g-PMMA at 100 Hz, 1000 Hz, and 20 kHz, respectively. In addition, in Fig. 7, the variations in conductivity of starch-g-PMMA/CdS composites with different content of CdS are shown. When Fig. 7 is examined, it was determined that the conductivity of composites improved due to increasing CdS. The electrical conductivity of the composites increased from 2.66 × 10–9 to 3.27 × 10–9 S/cm when the amount of CdS was increased from 5 to 15 wt% at 1-kHz frequency. This increase in conductivity, even at low concentrations, attributed to the enhanced interfacial polarization or the Maxwell–Wagner–Sillars effect [45, 46]. This effect occurs on heterogeneous surfaces with different conductivities due to charge accumulation at the interfaces. When the CdS content in the polymer is low, there is no interaction between the metal particles, and they are well dispersed. Since the CdS particles are well separated, there will be no coupling between them under the applied electric field. As a result, the probability of electrons jumping to adjacent molecule electrons will be very low [45]. As the CdS content increases, the number of particles per unit volume and the interfacial area increase, while the interparticle distance (IPD) decreases. This increases the average polarization associated with the particles and the coupling between adjacent grains [46, 47] and contributes more to the conductivity of the composites. Many studies have been conducted investigating the effect of CdS on the electrical conductivity of polymers. Our results appear to be in agreement with these studies. Sheng et al. prepared cadmium sulfide/poly(methyl methacrylate) (CdS/PMMA) nanocomposite films with different content of CdS by a new solution casting method. The conductivity result showed that increasing quantity of CdS in PMMA matrix has resulted in the increasing of electrical conductivity, which can be associated to the increased contact and Cd2+ ion transference between the CdS particles [48]. Hmar et al. noted that, although pure PVA behaves like an insulating polymer with conductivity 10−10–10−11 S/cm, CdS-PVA nanocomposite shows n-type semiconducting nature due to the presence of interconnected CdS nanostructures [58,59,60]. σac values are summarized in Table 3.
Impedance
Impedance (Z) is the measure of internal resistance, alternating current resistance and the resistance of the circuit to the passage of current when voltage is applied to a system. This value emerges from the response of materials to alternating electric current stimulation [48]. Z measurement is one of the powerful techniques used to perform and characterize charge transfer in complex materials [49]. Z measurements of the starch-g-PMMA and its composites were taken between 100 Hz and 20 kHz. These values were plotted as a function of frequency and is shown in Fig. 8. Z decreases with frequency up to ∼6 kHz, at frequencies above this frequency, dependence decreases and values remain almost constant. The decrease in Z may be due to increased segmental mobility due to reasons such as bond rupturing in the molecule or increase in free volume [49]. It can be seen that Z is lower for the composites which contain CdS than starch-g-PMMA. The impedance results of the composites are given in Table 3.
Conclusions
Starch-g-PMMA/CdS polymer composite was successfully synthesized and produced. According to X-ray diffraction patterns and energy-dispersive spectroscopy results, the presence of metal in the polymer composite was confirmed. The dielectric and electrical properties of the composites containing CdS at different content for electronic applications were investigated. The obtained values of the dielectric constant (ε′) of the prepared samples were shifted from 5.44 (starch-g-PMMA) to 14.63 (starch-g-PMMA 15%CdS) at 10-kHz frequency. The AC conductivity of the composites increased with an increase in the CdS amount, which can be assigned to the production of charge transfer complexes. The maximum conductivity was found to be 8.87 × 10–7 for starch-g-PMMA 15%CdS composite at 10 kHz. Additionally, the addition of CdS also improved the dielectric loss of the composites. The impedance of composites were evaluated from the dielectric data. Since CdS provides a significant improvement in the dielectric properties of starch-g-PMMA, it has given this polymer new properties and increased their usage areas. Such materials have a wide range of applications, from dielectric capacitors to parts of mobile phones, satellite communication systems, and microwave devices.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Al-Muntaser AA, Abdelghany AM, Abdelrazek EM, Elshahawy AG (2020) Enhancement of optical and electrical properties of PVC/PMMA blend films doped with Li4Ti5O12 nanoparticles. J Market Res 9(1):789–797
Alsulami QA, Rajeh A (2022) Structural, thermal, optical characterizations of polyaniline/polymethyl methacrylate composite doped by titanium dioxide nanoparticles as an application in optoelectronic devices. Opt Mater 123:111820
Zhu F (2018) Modifications of starch by electric field based techniques. Trends Food Sci Technol 75:158–169
Torgut G, Gürler N (2021) Grafen Katkılı Nişasta Filmlerinin Dielektrik Özelliklerinin Geniş Frekans Aralığında İncelenmesi. J Inst Sci Technol 11(2):1393–1401
Gürler N (2023) Development of chitosan/gelatin/starch composite edible films incorporated with pineapple peel extract and aloe vera gel: Mechanical, physical, antibacterial, antioxidant, and sensorial analysis. Polym Eng Sci 63(2):426–440
Gürler N, Paşa S, Erdoğan Ö, Cevik O (2023) Physicochemical properties for food packaging and toxicity behaviors against healthy cells of environmentally friendly biocompatible starch/citric acid/polyvinyl alcohol biocomposite films. Starch-Stärke 75(3–4):2100074
Çankaya N (2016) Synthesis of graft copolymers onto starch and its semiconducting properties. Results Phys 6:538–542
Qudsieh IY, Fakhru’l-Razi A, Muyibi SA, Ahmad MB, Rahman MA, Yunus ZW, W. M. (2004) Preparation and characterization of poly (methyl methacrylate) grafted sago starch using potassium persulfate as redox initiator. J Appl Polym Sci 94(5):1891–1897
Khiar AA, Arof AK (2010) Conductivity studies of starch-based polymer electrolytes. Ionics 16:123–129
Yusof YM, Shukur MF, Illias HA, Kadir MFZ (2014) Conductivity and electrical properties of corn starch–chitosan blend biopolymer electrolyte incorporated with ammonium iodide. Phys Scr 89(3):035701
Gürler N, Paşa S, Alma MH, Temel H (2020) The fabrication of bilayer polylactic acid films from cross-linked starch as eco-friendly biodegradable materials: synthesis, characterization, mechanical and physical properties. Eur Polym J 127:109588
Liu P, Ma C, Li Y, Wang L, Wei L, Yan Y, Xie F (2020) Facile preparation of eco-friendly, flexible starch-based materials with ionic conductivity and strain-responsiveness. ACS Sustain Chem Eng 8(51):19117–19128
Pongviratchai P, Park JW (2007) Electrical conductivity and physical properties of surimi–potato starch under Ohmic heating. J Food Sci 72(9):E503–E507
Ayala G, Agudelo A, Vargas R (2012) Effect of glycerol on the electrical properties and phase behavior of cassava starch biopolymers. Dyna 79(171):138–147
Bin-Dahman OA, Rahaman M, Khastgir D, Al-Harthi MA (2018) Electrical and dielectric properties of poly (vinyl alcohol)/starch/graphene nanocomposites. Can J Chem Eng 96(4):903–911
Abdelghany AM, Oraby AH, Asnag GM (2019) Structural, thermal and electrical studies of polyethylene oxide/starch blend containing green synthesized gold nanoparticles. J Mol Struct 1180:15–25
Morales-Sanchez E, Figueroa JDC, Gaytan-Martínez M (2009) Wet method for measuring starch gelatinization temperature using electrical conductivity. J Food Sci 74(7):E382–E385
Bauer BA, Knorr D (2004) Electrical conductivity: a new tool for the determination of high hydrostatic pressure-induced starch gelatinisation. Innov Food Sci Emerg Technol 5(4):437–442
Wang WC, Sastry SK (1997) Starch gelatinization in Ohmic heating. J Food Eng 34(3):225–242
Chaiwanichsiri S, Ohnishi S, Suzuki T, Takai R, Miyawaki O (2001) Measurement of electrical conductivity, differential scanning calorimetry and viscosity of starch and flour suspensions during gelatinisation process. J Sci Food Agric 81(15):1586–1591
El-Gamal AA (2023) Optical and electrical properties of polystyrene/poly-methyl methacrylate polymeric blend filled with semiconductor and insulator nanofillers. Phys Stat Solidi (RRL) Rapid Res Lett. https://doi.org/10.1002/pssr.202300145
Soumya S, Mohamed AP, Mohan K, Ananthakumar S (2015) Enhanced near-infrared reflectance and functional characteristics of Al-doped ZnO nano-pigments embedded PMMA coatings. Sol Energy Mater Sol Cells 143:335–346
Pan Y, Liu X, Kaschta J, Hao X, Liu C, Schubert DW (2017) Viscoelastic and electrical behavior of poly (methyl methacrylate)/carbon black composites prior to and after annealing. Polymer 113:34–38
Nayak D, Choudhary RB (2019) Augmented optical and electrical properties of PMMA-ZnS nanocomposites as emissive layer for OLED applications. Opt Mater 91:470–481
Nafee SS, Hamdalla TA, Darwish AAA (2020) Studies of the morphology and optical properties of nano erbium oxide embedded in PMMA matrix. Opt Laser Technol 129:106282
Yang B, Pan Y, Yu Y, Wu J, Xia R, Wang S, Tu Y (2020) Filler network structure in graphene nanoplatelet (GNP)-filled polymethyl methacrylate (PMMA) composites: From thermorheology to electrically and thermally conductive properties. Polymer Test 89:106575
Beygisangchin M, Abdul Rashid S, Shafie S, Sadrolhosseini AR, Lim HN (2021) Preparations, properties, and applications of polyaniline and polyaniline thin films—a review. Polymers 13(12):2003
Morsi MA, Rajeh A, Al-Muntaser AA (2019) Reinforcement of the optical, thermal and electrical properties of PEO based on MWCNTs/Au hybrid fillers: nanodielectric materials for organoelectronic devices. Compos B Eng 173:106957
Rodríguez-Fragoso P, de la Cruz GG, Tomas SA, Zelaya-Angel O (2010) Optical characterization of CdS semiconductor nanoparticles capped with starch. Appl Surf Sci 257(2):581–584
Lahariya V, Dhoble SJ (2022) Development and advancement of undoped and doped zinc sulfide for phosphor application. Displays 74:102186
Koteswararao J, Abhishek R, Satyanarayana SV, Madhu GM, Venkatesham V (2016) Influence of cadmium sulfide nanoparticles on structural and electrical properties of polyvinyl alcohol films. Express Polym Lett 10(11):883–894
Uddin I, Abzal SM, Kalyan K, Janga S, Patel R, Dash JK (2023) Starch-assisted stable synthesis of CdS nanoparticles for enhanced electrical and optical properties. J Electron Mater 52(3):1710–1716
Abdel-Galil A, Balboul MR, Ali HE (2020) Synthesis and characterization of γ-irradiated cadmium sulfide/polyvinyl alcohol nanocomposites films. J Electron Mater 49:2222–2232
Ingle RV, Shaikh SF, Kaur J, Ubaidullah M, Pandit B, Pathan HM (2023) Optical and electronic properties of colloidal cadmium sulfide. Mater Sci Eng B 294:116487
İyibakanlar G, Oktay A (2007) Bazı Polimerlerin Dielektrik Özelliklerinin Frekansla Değişimlerinin İncelenmesi. Havacılık Ve Uzay Teknolojileri Dergisi 3(1):11–19
Thakur VK, Gupta RK (2016) Recent progress on ferroelectric polymer-based nanocomposites for high energy density capacitors: synthesis, dielectric properties, and future aspects. Chem Rev 116:4260–4317
Zhu L (2014) Exploring strategies for high dielectric constant and low loss polymer dielectrics. J Phys Chem Lett 5:3677–3687
Wang Q, Che J, Wu W, Hu Z, Liu X, Ren T, Chen Y, Zhang J (2023) Contributing factors of dielectric properties for polymer matrix composites. Polymers 15:590
Psarras GC, Manolakaki E, Tsangarris GM (2002) Composites A 33:375–384
Ghany S, Salam AE, Nasr GM (2000) J Appl Polym Sci 77:1816–1821
Qureshi A, Mergen A, Eroğlu MS, Singh NL, Güllüoğlu A (2008) Dielectric properties of polymer composites filled with different metals. J Macromol Sci w, Part A Pure Appl Chem 45:462–469
Li Y, Krentz TM, Wang L, Benicewicz BC, Schadler LS (2014) Ligand Engineering of polymer nanocomposites: from the simple to the complex. ACS Appl Mater Interfaces 6:6005–6021
Yuan J-K, Yao S-H, Dang Z-M, Sylvestre A, Genestoux M, Giant BJ (2011) Dielectric permittivity nanocomposites: realizing true potential of pristine carbon nanotubes in polyvinylidene fluoride matrix through an enhanced interfacial interaction. J Phys Chem C 115:5515–5521
Norah Algethami A, Rajeh HM, Ragab AE, Tarabiah, and Fatma Gami, (2022) Characterization, optical, and electrical properties of chitosan/polyacrylamide blend doped silver nanoparticles. J Mater Sci: Mater Electron 33:10645–10656
Deepa KS, Gopika MS, James J (2013) Influence of matrix conductivity and Coulomb blockade effect on the percolation threshold of insulator–conductor composites. Compos Sci Technol 78:18–23
Singh V, Kulkarni AR, Rama Mohan TR (2003) Dielectric properties of aluminum– epoxy composites. J Appl Pol Sci 90:3602–3608
Deepa KS, Sebastian MT, James J (2007) Effect of interparticle distance and interfacial area on the properties of insulator–conductor composites. Appl Phys Lett 91:202904
Yakuphanoglu F, Okutan M, Zhuang Q, Han Z (2005) The dielectric spectroscopy and surface morphology studies in a new conjugated polymer poly(benzobisoxazole-2,6-diylvinylene). Physica B 365:13–19
Dinesh P, Renukappa NM, Siddaramaiah, (2010) Impedance and susceptance characterization of multiwalled carbon nanotubes with high density polyethylene-carbon black nanocomposites. Integr Ferroelectr 116:128–136
Koran K, Özen F, Torğut G, Pıhtılı G, Çil E, Görgülü AO, Arslan M (2014) Synthesis, characterization and dielectric properties of phosphazenes containing chalcones. Polyhedron 79(5):213–220
Torğut G, Gürler N (2021) Nanofiller reinforced biodegradable PHA/PLA composites: physico-chemical, thermal and dielectric properties. J Polym Res 28(452):1–11
Mondal SP, Mullick H, Lavanya T, Dhar A, Ray SK (2007) Optical and dielectric properties of junctionlike CdS nanocomposites embedded in polymer matrix. J Appl Phys 102:064305
Kalandaragh YA (2010) Dielectric properties of CdS-PVA nanocomposites prepared by ultrasound-assisted method. Optoelectron Adv Mater Rapid Commun 4(11):1655–1658
Bhunia R, Ghosh D, Ghosh B, Hussain S, Bhara R, Pal AK (2015) Some aspects of microstructural and dielectric properties of nanocrystalline CdS/poly (vinylidene fluoride) composite thin films. Polym Int 64:924–934
Khurana K, Jaggi N (2020) Modifications in structural, optical, and dielectric properties of CdS nanostructures: role of different solvents. J Mater Sci: Mater Electron 31:10334–10346
Althubiti NA, Al-Harbi N, Sendi RK, Atta A, Henaish AMA (2023) Surface characterization and electrical properties of low energy irradiated PANI/PbS polymeric nanocomposite materials. Inorganics 11:74
Althubiti NA, Abdelhamied MM, Abdelreheem AM, Atta A (2022) Oxygen irradiation induced modification on the linear and nonlinear optical behavior of flexible MC/PANI/Ag polymeric nanocomposite films. Inorg Chem Commun 137:109229
Sheng CK, Amin KAM, Hong LL, Hassan MF, Ismail M (2017) Investigation of morphological, structural and electrical properties of Cds/PMMA nanocomposite film prepared by solution casting method. Int J Electrochem Sci 12(11):10023–10031
Mahendia S, Tomar AK, Kumar S (2010) Electrical conductivity and dielectric spectroscopic studies of PVA–Ag nanocomposite films. J Alloy Compd 508:406–411
Hmar JJL, Majumder T, Roy JN, Mondal SP (2015) Electrical and photoelectrochemical characteristics of flexible CdS nanocomposite/conducting polymer heterojunction. Mater Sci Semicond Process 40:145–151
Yasmeen U, Haq F, Kiran M, Farid A, Ullah N, Aziz T, Ullah I (2022) Synthesis of starch-grafted polymethyl methacrylate via free radical polymerization reaction and its application for the uptake of methylene blue. Molecules 27(18):5844
Atta A, Alotaibi BM, Abdelhamied MM (2022) Structural characteristics and optical properties of methylcellulose/polyaniline films modified by low energy oxygen irradiation. Inorg Chem Commun 141:109502
Althubiti NA, Atta A, Al-Harbi N, Sendi RK, Abdelhamied MM (2023) Structural, characterization and linear/nonlinear optical properties of oxygen beam irradiated PEO/NiO composite films. Opt Quant Electron 55(4):348
Abdeltwab E, Atta A (2021) Influence of ZnO nanoadditives on the structural characteristics and dielectric properties of PVA. Int J Mod Phys B 35(30):2150310
Rahman NA, Hanifah SA, Mobarak NN, Ahmad A, Ludin NA, Bella F, Su’ait MS (2021) Chitosan as a paradigm for biopolymer electrolytes in solid-state dye-sensitised solar cells. Polymer 230:124092
Abdulwahid RT, Aziz SB, Kadir MF (2023) Environmentally friendly plasticized electrolyte based on chitosan (CS): Potato starch (PS) polymers for EDLC application: steps toward the greener energy storage devices derived from biopolymers. J Energy Storage 67:107636
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
GT helped in data curation, writing—original draft, and methodology and NG was involved in data curation, conceptualization, methodology, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Torğut, G., Gürler, N. Enhanced impedance, electrical conductivity, dielectric properties for colloidal starch-g-poly (methyl methacrylate) supported with semiconductor cadmium sulfide. Polym. Bull. (2024). https://doi.org/10.1007/s00289-023-05125-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-023-05125-5