Abstract
Albumin is an abundant protein in nature with several biological functions. In the human body, both in health and in illness, its transport function is highlighted by the binding to medicinal drugs and consequent distribution in the bloodstream to the site of action. This is particularly relevant for anticancer treatments, since this protein accumulates in the tumor microenvironment to supply the energetic demands of cancer cells. Different spectroscopy, thermodynamic and in silico studies techniques can be employed to verify how albumin binds to ligands by using either human serum albumin (HSA) or bovine serum albumin (BSA) due to their structural similarity. There is an increasing tendency to use albumin in analyses of absorption, distribution, metabolism and excretion (ADME) properties of anticancer molecules, which further demonstrated the promising character of this investigation for proposing new drugs.











Similar content being viewed by others
References
Ràfols C, Zarza S, Bosch E. Molecular interactions between some non-steroidal anti-inflammatory drugs (NSAID׳ s) and bovine (BSA) or human (HSA) serum albumin estimated by means of isothermal titration calorimetry (ITC) and frontal analysis capillary electrophoresis (FA/CE). Talanta. 2014;130:241–50.
Abdelhameed AS, Bakheit AH, Almutairi FM, AlRabiah H, Kadi AA. Biophysical and in silico studies of the interaction between the anti-viral agents acyclovir and penciclovir, and human serum albumin. Molecules. 2017;22:1906–11. https://doi.org/10.3390/molecules22111906.
Zhang HX, Xiong HX, Li LW. Investigation on the protein-binding properties of icotinib by spectroscopic and molecular modeling method. Spectrochim Acta A. 2016;161:88–94. https://doi.org/10.1016/j.saa.2016.02.014.
Filho FA, Souza TF, Ribeiro AG, Alves JE, Oliveira JF, Souza TR, et al. Topoisomerase inhibition and albumin interaction studies of acridine thiosemicarbazone derivatives. Int J Biol Macromol. 2019;138:582–9.
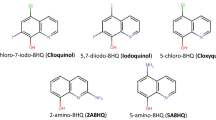
Ribeiro AG, Almeida SMV, Oliveira JF, Souza TRCL, Santos KL, Albuquerque APB, et al. Novel 4-quinoline-thiosemicarbazone derivatives: synthesis, antiproliferative activity, in vitro and in silico biomacromolecule interaction studies and topoisomerase inhibition. Eur. J Med Chem. 2019;182:111592 https://doi.org/10.1016/j.ejmech.2019.111592.
Pawar SK, Jaldappagari S. Probing the mechanism of interaction of metoprolol succinate with human serum albumin by spectroscopic and molecular docking analysis. Luminescence. 2017;32:942–51. https://doi.org/10.1002/bio.3275.
Villarreal W, Colina-Vegas L, Visbal G, Corona O, Corrêa RS, Ellena J, et al. Copper (I)–phosphine polypyridyl complexes: synthesis, characterization, DNA/HSA binding study, and antiproliferative activity. Inorg Chem. 2017;56:3781–93. https://doi.org/10.1021/acs.inorgchem.6b02419.
Xu H, Yao N, Xu H, Wang T, Li G, Li Z. Characterization of the interaction between eupatorin and bovine serum albumin by spectroscopic and molecular modeling methods. Int J Mol Sci. 2013;14:14185–203. https://doi.org/10.3390/ijms140714185.
Rabbani G, Lee EJ, Ahmad K, Baig MH, Choi I. Binding of tolperisone hydrochloride with human serum albumin: effects on the conformation, thermodynamics, and activity of HSA. Mol Pharm. 2018;15:1445–56. https://doi.org/10.1021/acs.molpharmaceut.7b00976.
Baig MH, Rahman S, Rabbani G, Imran M, Ahmad K, Choi I. Multi-spectroscopic characterization of human serum albumin binding with cyclobenzaprine hydrochloride: insights from biophysical and in silico approaches. Int J Mol Sci. 2019;2:662 https://doi.org/10.3390/ijms20030662.
Nurdiansyah R, Rifa’i M. A comparative analysis of serum albumin from different species to determine a natural source of albumin that might be useful for human therapy. J Taibah Univ Med Sci. 2016;11:243–9.
Fu XB, Weng GT, Liu DD, Le XY. Synthesis, characterization, DNA binding and cleavage, HSA interaction and cytotoxicity of a new copper (II) complex derived from 2-(2′-pyridyl) benzothiazole and glycylglycine. J Photochem Photobio A Chem. 2014;276:83–95.
Ebrahimipour SY, Mohamadi M, Mahani MT, Simpson J, Mague JT, Sheikhshoaei I. Synthesis and structure elucidation of novel salophen-based dioxo-uranium (VI) complexes: In-vitro and in-silico studies of their DNA/BSA-binding properties and anticancer activity. Eur J Med Chem. 2017;140:172–86. https://doi.org/10.1016/j.ejmech.2017.08.068.
Ćoćić D, Jovanović S, Radisavljević S, Korzekwa J, Scheurer A, Puchta R, et al. New monofunctional platinum (II) and palladium (II) complexes: Studies of the nucleophilic substitution reactions, DNA/BSA interaction, and cytotoxic activity. J Inorg Biochem. 2018;189:91–102.
Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS J. 2008;582:1783–7.
Fanali G, Di Mais A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Asp Med. 2012;33:209–90.
Nahon JL, Tratner I, Poliard A, Presse F, Poiret M, Gal A, et al. Albumin and alpha-fetoprotein gene expression in various non-hepatic rat tissues. J Biol Chem. 1988;263:11436–42.
Yoshida K, Seto-Ohshima A, Sinohara H. Sequencing of cDNA encoding serum albumin and its extrahepatic synthesis in the Mongolian gerbil, Meriones unguiculatus. DNA Res. 1997;4:351–4.
Dodson CS, Rengarajan K, Gewant HD, Stodulkova E, Nguyen HT, Boatright JH, et al. Extra-hepatic expression of serum albumin mRNA in mouse retina. Curr Eye Res. 2001;22:182–9.
Carvalho JR, Machado MV. New insights about albumin and liver disease. Ann Hepatol. 2018;17:547–60.
Varanko A, Saha S, Chilkoti A. Recent trends in protein and peptide-based biomaterials for advanced drug delivery. Adv Drug Deliv Ver. 2020;55:2–55.
Mazzaferro EM, Rudloff E, Kirby R. The role of albumin replacement in the critically ill veterinary patient. J Vet Emerg Crit Car. 2002;12:113–24.
Era S, Ashida H, Nagaoka S, Inouye H, Sogami M. CD‐resolved secondary structure of bovine plasma albumin in acid-induced isomerization. Int J Pept Protein Res. 1983;22:333–40.
Korch-Weser J, Sellers EM. Binding of drugs to serum albumin (first of two parts). New Eng J Med. 1976;294:311–6.
Dugaiczyk A, Law SW, Dennison OE. Nucleotide sequence and the encoded amino acids of human serum albumin mRNA. Proc Nat Acad Sci. 1982;79:71–5.
Wang Y, Yu H, Shi X, Luo Z, Lin D, Huang M. Structural mechanism of ring-opening reaction of glucose by human serum albumin. J Biol Chem. 2013;288:15980–7.
Sun YE, Wang WD. Study on the interaction of bioactive compound S-allyl cysteine from garlic with serum albumin. J Food Drug Anal. 2017;25:385–90.
AlQahtani AD, O’Connor D, Domling A, Goda SK. Strategies for the production of long-acting therapeutics and efficient drug delivery for cancer treatment. Biomed Pharmacother. 2019;113:108750.
Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, et al. The major histocompatibility complex–related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197:315–22.
Nilsen J, Trabjerg E, Grevys A, Azevedo C, Brennan SO, Stensland M, et al. An intact C-terminal end of albumin is required for its long half-life in humans. Commun Biol. 2020;3:1–11.
Hoogenboezem EN, Duvall CL. Harnessing albumin as a carrier for cancer therapies. Adv Drug Deliv Ver. 2018;130:73–89.
Moujaess E, Fakhoury M, Assi T, Elias H, El Karak F, Ghosn M, et al. The Therapeutic use of human albumin in cancer patients’ management. Crit Rev Oncol Hemat. 2017;120:203–9. https://doi.org/10.1016/j.critrevonc.2017.11.008.
Taverna M, Marie AL, Mira JP, Guidet B. Specific antioxidant properties of human serum albumin. Ann Intensive Care. 2013;3:4 https://doi.org/10.1186/2110-5820-3-4.
Komrokji RS, Corrales‐Yepez M, Kharfan‐Dabaja MA, Al Ali NH, Padron E, Rollison DE, et al. Hypoalbuminemia is an independent prognostic factor for overall survival in myelodysplastic syndromes. Am J Hematol. 2012;87:1006–9.
Fang CM, Ku MC, Chang CK, Liang HC, Wang TF, Wu CH, et al. Identification of endogenous site-specific covalent binding of catechol estrogens to serum proteins in human blood. Toxicol Sci. 2015;148:433–42. https://doi.org/10.1093/toxsci/kfv190.
Iwao Y, Ishima Y, Yamada J, Noguchi T, Kragh‐Hansen U, Mera K, et al. Quantitative evaluation of the role of cysteine and methionine residues in the antioxidant activity of human serum albumin using recombinant mutants. IUBMB Life. 2012;64:450–4. https://doi.org/10.1002/iub.567.
Suo Z, Xinnuo X, Qiaomei S, Ludan Z, Peixiao T, Quan H, et al. Investigation on the interaction of dabrafenib with human serum albumin by using combined experiment and molecular dynamics simulation: exploring the binding mechanism, esterase-like activity, and antioxidant activity. Mol Pharm. 2018;15:5637–45. https://doi.org/10.1021/acs.molpharmaceut.8b00806.
Lakshmi P, Mondal M, Ramadas K, Natarajan S. Molecular interaction of 2, 4-diacetylphloroglucinol (DAPG) with human serum albumin (HSA): the spectroscopic, calorimetric and computational investigation. Spectrochim Acta A Mol Biomol Spectrosc. 2017;183:90–102.
FDA. Medication Guide TANZEUM™ (TAN-zee-um) (albiglutide) for injection, for subcutaneous use. 2015. https://www.fda.gov/. Accessed 11 Dec 2020.
Rendell MS. Albiglutide: a unique GLP-1 receptor agonist. Expert Opin Biol Ther. 2016;16:1557–69.
ALBUMEDIX, Veltis® - Enhancing the body’s natural drug delivery system. Albumedix© Copyright 2018. https://albumedix.com/technology/veltis/.
Lambrinidis G, Vallianatou T, Tsantili-Kakoulidou A. In vitro, in silico and integrated strategies for the estimation of plasma protein binding. A review. Adv Drug Deliv Ver. 2015;86:27–45.
Smith DA, Di L, Kerns EH. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Disco. 2010;9:929–39.
Pilati D, Howard KA. Albumin-based drug designs for pharmacokinetic modulation. Expert Opin Drug Metab Toxicol. 2020;16:783–95. https://doi.org/10.1080/17425255.2020.1801633.
Almeida SMV, Ribeiro AG, de Lima Silva GC, Alves JEF, Beltrão EIC, de Oliveira JF, et al. DNA binding and Topoisomerase inhibition: How can these mechanisms be explored to design more specific anticancer agents? Biomed Pharmacother. 2017;96:1538–56.
Matos MJ. Learning from nature: the role of albumin in drug delivery. Future Med Chem. 2018;10:983–5.
Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Rel. 2008;132:171–83.
Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin—more than just a serum protein. Front Physiol. 2014;5:299.
Sanches M, D’Angelo I, Jaramillo M, BaardsnesJ, Zwaagstra J, Schrag J, et al. AlbuCORE: an albumin-based molecular scaffold for multivalent biologics design. J Mabs. 2020;12:1802188.
Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–803.
Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30:2055–62.
Iwamoto T. Clinical application of drug delivery systems in cancer chemotherapy: review of the efficacy and side effects of approved drugs. Biol Pharm Bull. 2013;36:715–8.
Palumbo R, Sottotetti F, Bernardo A. Targeted chemotherapy with nanoparticle albumin-bound paclitaxel (nab-paclitaxel) in metastatic breast cancer: which benefit for which patients? Ther Adv Med Oncol. 2016;8:209–29.
Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–44.
Ishima Y, Maruyama T. Human serum albumin as carrier in drug delivery systems. Yakugaku Zasshi. 2016;136:39–47.
Battogtokh G, Gotov O, Kang JH, Cho J, Jeong TH, Chimed G, et al. Triphenylphosphine-docetaxel conjugate-incorporated albumin nanoparticles for cancer treatment. Nanomedicine. 2018;13:325–38.
Greco F, Vicent MJ. Combination therapy: opportunities and challenges for polymer–drug conjugates as anticancer nanomedicines. Adv Drug Deliv Rev. 2009;61:1203–13.
Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Disco Today. 2012;17:1044–52.
Cao X, Luo J, Gong T, Zhang ZR, Sun X, Fu Y. Coencapsulated doxorubicin and bromotetrandrine lipid nanoemulsions in reversing multidrug resistance in breast cancer in vitro and in vivo. Mol Pharm. 2015;12:274–86.
Gou Y, Zhang Z, Li D, Zhao L, Cai M, Sun Z, et al. HSA-based multi-target combination therapy: regulating drugs’ release from HSA and overcoming single drug resistance in a breast cancer model. Drug Deliv. 2018;25:321–9.
Garmann D, Warnecke A, Kalayda GV, Kratz F, Jaehde U. Cellular accumulation and cytotoxicity of macromolecular platinum complexes in cisplatin-resistant tumor cells. J Control Rel. 2008;131:100–6.
Zhao D, Zhang H, Tao W, Wei W, Sun J, He Z. A rapid albumin-binding 5-fluorouracil prodrug with a prolonged circulation time and enhanced antitumor activity. Biomater Sci. 2017;5:502–10. https://doi.org/10.1039/C6BM00884D.
Pes L, Koester SD, Magnusson JP, Chercheja S, Medda F, Ajaj KA, et al. Novel auristatin E-based albumin-binding prodrugs with superior anticancer efficacy in vivo compared to the parent compound. J Control Rel. 2019;296:81–92. https://doi.org/10.1016/j.jconrel.2019.01.010.
Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level. Oncol Rep. 2003;10:1663–82.
Mukherjee S, Mitra I, Das P, Misini B, Linert W, Moi SC. In vitro DNA/BSA binding, anticancer and normal cell activity of Pd (II) complexes: substitution behaviour and computational study. Chem Sel. 2018;3:3871–85.
Sribalan R, Banuppriya G, Kirubavathi M, Padmini V. Synthesis, biological evaluation and in silico studies of tetrazole-heterocycle hybrids. J Mol Struct. 2019;1175:577–86.
Keri RS, Chand K, Budagumpi S, Somappa SB, Patil SA, Nagaraja BM. An overview of benzo [b] thiophene-based medicinal chemistry. Eur J Med Chem. 2017;138:1002–33.
Manjal SK, Kaur R, Bhatia R, Kumar K, Singh V, Shankar R, et al. Synthetic and medicinal perspective of thiazolidinones: a Review. Bioorg Chem. 2017;75:406–23.
Asadizadeh S, Amirnasr M, Tirani FF, Mansouri A, Schenk K. DNA-BSA interaction, cytotoxicity and molecular docking of mononuclear zinc complexes with reductively cleaved N2S2 Schiff base ligands. Inorg Chim Acta. 2018;483:310–20. https://doi.org/10.1016/j.ica.2018.08.037.
Baltazar CJ, Mun R, Tajmir-Riahi HA, Bariyanga J. Spectroscopic studies on the interaction of mimosine with BSA and DNA. J Mol Struct. 2018;1161:273–8. https://doi.org/10.1016/j.molstruc.2018.01.039.
Mohamadi M, Hassankhani A, Ebrahimipour SY, Torkzadeh-Mahani M. In vitro and in silico studies of the interaction of three tetrazoloquinazoline derivatives with DNA and BSA and their cytotoxicity activities against MCF-7, HT-29 and DPSC cell lines. Int J Biol Macromol. 2017;94:85–95. https://doi.org/10.1016/j.ijbiomac.2016.09.113.
Grandis RA, Rone A, de Camargo MS, Da Silva MM, Lopes ÉO, Padilha EC, et al. Human topoisomerase inhibition and DNA/BSA binding of Ru (II)–SCAR complexes as potential anticancer candidates for oral application. BioMetals. 2017;30:321–34. https://doi.org/10.1007/s10534-017-0008-z.
Gouveia RG, Ribeiro AG, Segundo MÂSP, de Oliveira JF, de Lima MDCA, de Lima Souza TRC, et al. Synthesis, DNA and protein interactions and human topoisomerase inhibition of novel Spiroacridine derivatives. Bioorg Med Chem. 2018;26:5911–21. https://doi.org/10.1016/j.bmc.2018.10.038.
Karami K, Alinaghi M, Amirghofran Z, Lipkowski J. Synthesis and characterization of two new trans palladium (II) complexes containing benzylamine ligand: DNA/BSA interactions, molecular docking and in vitro cytotoxic activity. Inorg Chim Acta. 2018;471:797–807. https://doi.org/10.1016/j.ica.2017.02.027.
Shen GF, Liu TT, Wang Q, Jiang M, Shi JH. Spectroscopic and molecular docking studies of binding interaction of gefitinib, lapatinib and sunitinib with bovine serum albumin (BSA). J Photochem Photobio B. 2015;153:380–90. https://doi.org/10.1016/j.jphotobiol.2015.10.023.
Prinsen BH. Albumin turnover: experimental approach and its application in health and renal diseases. Clin Chim Acta. 2004;347:1–14.
Bernardi M, Ricci CS, Zaccherini G. Role of human albumin in the management of complications of liver cirrhosis. J Clin Exp Hepatol. 2014;4:302–11.
Levitt DG, Levitt MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016;9:229.
Peters JT. All about albumin. Academic Press. Elsevier. 1995. ISBN 978-0-12-552110-9. https://doi.org/10.1016/B978-0-12-552110-9.X5000-4
Hawkins JW, Dugaiczyk A. The human serum albumin gene: structure of a unique locus. Gene. 1982;19:55–8.
Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211–9.
Wardell M, Wang Z, Ho JX, Robert J, Ruker F, Ruble J, et al. The atomic structure of human methemalbumin at 1.9 Å. Biochem Biophys Res Commun. 2002;291:813–9. https://doi.org/10.1006/bbrc.2002.6540.
Petitpas I, Petersen CE, Ha CE, Bhattacharya AA, Zunszain PA, Ghuman J, et al. Structural basis of albumin–thyroxine interactions and familial dysalbuminemic hyperthyroxinemia. PNAS. 2003;100:6440–5. https://doi.org/10.1073/pnas.1137188100.
Zunszain PA, Ghuman J, McDonagh AF, Curry S. Crystallographic analysis of human serum albumin complexed with 4Z, 15E-bilirubin-IXα. J Mol Biol. 2008;381:394–406. https://doi.org/10.1016/j.jmb.2008.06.016.
Yang F, Bian C, Zhu L, Zhao G, Huang Z, Huang M. Effect of human serum albumin on drug metabolism: structural evidence of esterase activity of human serum albumin. J Struct Biol. 2007;157:348–55. https://doi.org/10.1016/j.jsb.2006.08.015.
Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, Curry S. Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol. 2005;353:38–52. https://doi.org/10.1016/j.jmb.2005.07.075.
Zhu L, Yang F, Chen L, Meehan EJ, Huang M. A new drug binding subsite on human serum albumin and drug–drug interaction studied by X-ray crystallography. J Struct Biol. 2008;162:40–49. https://doi.org/10.1016/j.jsb.2007.12.004.
Schmidt S, Gonzalez D, Derendorf H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99:1107–22. https://doi.org/10.1002/jps.21916.
Mera K, Anraku M, Kitamura K, Nakajou K, Maruyama T, Otagiri M. The structure and function of oxidized albumin in hemodialysis patients: Its role in elevated oxidative stress via neutrophil burst. Biochem Biophys Res Commun. 2005;334:1322–8.
Kawakami A, Kubota K, Yamada N, Tagami U, Takehana K, Sonaka I, et al. Identification and characterization of oxidized human serum albumin: A slight structural change impairs its ligand-binding and antioxidante functions. FEBS J. 2006;273:3346–57.
Rao H, Qi W, Su R, He Z, Peng X. Mechanistic and conformational studies on the interaction of human serum albumin with rhodamine B by NMR, spectroscopic and molecular modeling methods. J Mol Liq. 2020;316:113889 https://doi.org/10.1016/j.molliq.2020.113889.
Chilom CG, Bacalum M, Stanescu MM, Florescu M. Insight into the interaction of human serum albumin with folic acid: A biophysical study. Spectrochim Acta A Mol Biomol Spectrosc. 2018;204:648–56. https://doi.org/10.1016/j.saa.2018.06.093.
Chugh H, Kumar P, Tomar V, Kaur N, Sood D, Chandra R. Interaction of Noscapine with human serum albumin (HSA): A spectroscopic and molecular modelling approach. J Photochem Photobio A. 2019;372:168–76. https://doi.org/10.1016/j.jphotochem.2018.12.001.
Yan X, Yuan D, Pan D. Interactions of bromocarbazoles with human serum albumin using spectroscopic methods. Molecules. 2018;23:3120 https://doi.org/10.3390/molecules23123120
Jia J, Wang Y, LiuY, Xiang Y. Exploration of interaction of canthaxanthin with human serum albumin by spectroscopic and molecular simulation methods. Luminescence. 2018;33:425–32. https://doi.org/10.1002/bio.3430.
Alsamamra H, Abusharkh S, Abuteir M. Comparative studies on the interaction of human and bovine serum albumins with vitamin C. Eur J Biophys. 2018;6:17–22. https://doi.org/10.11648/j.ejb.20180601.13.
Arumugam SS, Subramanian N, Malaichamy I. New insights into the dimerization and site-specific cooperative interaction of Azure B with model transport proteins by spectroscopic and computational studies. J Photochem Photobio B. 2016;164:212–25. https://doi.org/10.1016/j.jphotobiol.2016.09.011.
Rudra S, Dasmandal S, Patra C, Patel BK, Paul S, Mahapatra A. Characterization of domain-specific interaction of synthesized dye with serum proteins by spectroscopic and docking approaches along with determination of in vitro cytotoxicity and antiviral activity. J Biomol Struct Dyn. 2018;36:3773–90. https://doi.org/10.1080/07391102.2017.1400468.
Chen Z, Wu Y, Zhu Z, Zhang Y. DNA cleavage, DNA/HSA binding study, and antiproliferative activity of a phenolate-bridged binuclear copper (II) complex. Biometals. 2019;32:227–40. https://doi.org/10.1007/s10534-019-00172-w.
Yinhua D, Foroughi MM, Aramesh-Boroujeni Z, Jahani S, Peydayesh M, Borhani F, et al. The synthesis, characterization, DNA/BSA/HSA interactions, molecular modeling, antibacterial properties, and in vitro cytotoxic activities of novel parent and niosome nano-encapsulated Ho (III) complexes. RSC Adv. 2020;10:22891–908. https://doi.org/10.1039/d0ra03436c.
Sarbadhikary P, Dube A. Spectroscopic investigations on the binding of an iodinated chlorin p 6-copper complex to human serum albumin. Photochem Photobio Sci. 2017;16:1762–70. https://doi.org/10.1039/c7pp00197e.
Aseman MD, Aryamanesh S, Shojaeifard Z, Hemmateenejad B, Nabavizadeh SM. Cycloplatinated (II) derivatives of mercaptopurine capable of binding Interactions with HSA/DNA. Inorg Chem. 2019;58:16154–70. https://doi.org/10.1021/acs.inorgchem.9b02696.
Banerjee A, Mohanty M, Lima S, Samanta R, Garribba E, Sasamori T, et al. Synthesis, structure and characterization of new dithiocarbazate based mixed ligand oxidovanadium (IV) complexes: DNA/HSA interaction, cytotoxic activity and DFT studies. N J Chem. 2020;44:10946–63. https://doi.org/10.1039/D0NJ01246G.
Beigoli S, Sharifi Rad A, Askari A, Assaran Darban R, Chamani J. Isothermal titration calorimetry and stopped flow circular dichroism investigations of the interaction between lomefloxacin and human serum albumin in the presence of amino acids. J Biomol Struct Dyn. 2019;37:2265–82. https://doi.org/10.1080/07391102.2018.1491421.
Tayyab S, Izzudin MM, Kabir MZ, Feroz SR, Tee WV, Mohamad SB, et al. Binding of an anticancer drug, axitinib to human serum albumin: Fluorescence quenching and molecular docking study. J Photochem Photobio B. 2016;162:386–94. https://doi.org/10.1016/j.jphotobiol.2016.06.049.
Khan AY, Suresh Kumar G. Exploring the binding interaction of potent anticancer drug topotecan with human serum albumin: Spectroscopic, calorimetric and fibrillation study. J Biomol Struct Dyn. 2018;36:2463–73. https://doi.org/10.1080/07391102.2017.1359671.
Gan N, Sun Q, Tang PY, Wu D, Xie T, Zhang Y, et al. Determination of interactions between human serum albumin and niraparib through multi-spectroscopic and computational methods. Spectrochim Acta A Mol Biomol Spectrosc. 2018A;206:126–34. https://doi.org/10.1016/j.saa.2018.07.100.
Moghadam NH, Salehzadeh S, Tanzadehpanah H, Saidijam M, Karimi J, Khazalpour S. In vitro cytotoxicity and DNA/HSA interaction study of triamterene using molecular modelling and multi-spectroscopic methods. J Biomol Struct Dyn. 2019;37:2242–53. https://doi.org/10.1080/07391102.2018.1489305.
Naik R, Jaldappagari S. Spectral and computational attributes: binding of a potent anticancer agent, dasatinib to a transport protein. J Mol Liq. 2019;293:111492 https://doi.org/10.1016/j.molliq.2019.111492.
Li X, Wang S. Study on the interaction of (+)-catechin with human serum albumin using isothermal titration calorimetry and spectroscopic techniques. N. J Chem. 2015;39:386–95.
Camargo CR, Caruso ÍP, Gutierrez SJC, Fossey MA, Barbosa Filho JM, Cornélio ML. Spectral and computational features of the binding between riparins and human serum albumin. Spectrochim Acta A Mol Biomol Spectrosc. 2018;190:81–8. https://doi.org/10.1016/j.saa.2017.08.068.
Wu D, Liu D, Zhang Y, Zhang Z, Li H. Unravelling the binding mechanism of benproperine with human serum albumin: a docking, fluorometric, and thermodynamic approach. Eur J Med Chem. 2018;146:245–50. https://doi.org/10.1016/j.ejmech.2018.01.064.
Karthikeyan S, Bharanidharan G, Kesherwani M, Mani KA, Srinivasan N, Velmurugan D, et al. Insights into the binding of thiosemicarbazone derivatives with human serum albumin: spectroscopy and molecular modelling studies. J Biomol Struct Dyn. 2016;34:1264–81. https://doi.org/10.1080/07391102.2015.1075905.
Sousa-Pereira D, Chaves OA, Dos Reis CM, de Oliveira MC, Sant’Anna CMR, Netto-Ferreira JC, et al. Synthesis and biological evaluation of N-aryl-2-phenyl-hydrazinecarbothioamides: experimental and theoretical analysis on tyrosinase inhibition and interaction with HSA. Bioorg Chem. 2018;81:79–87. https://doi.org/10.1016/j.bioorg.2018.07.035.
Chaves OA, de Lima Santos MR, de Oliveira MC, Sant’Anna CMR, Ferreira RC, Echevarria A, et al. Synthesis, tyrosinase inhibition and transportation behavior of novel β-enamino thiosemicarbazide derivatives by human serum albumin. J Mol Liq. 2018;254:280–90. https://doi.org/10.1016/j.molliq.2018.01.083.
Rahman S, Rehman MT, Rabbani G, Khan P, AlAjmi MF, Hassan M, et al. Insight of the interaction between 2, 4-thiazolidinedione and human serum albumin: a spectroscopic, thermodynamic and molecular docking study. Int J Mol Sci. 2019;20:2727 https://doi.org/10.3390/ijms20112727.
Chaves OA, Calheiro TP, Netto-Ferreira JC, de Oliveira MC, Franceschini SZ, de Salles CMC, et al. Biological evaluation of BF2-naphthyridine compounds: tyrosinase and acetylcholinesterase activity, CT-DNA and HSA binding property evaluations. Int J Biol Macromol. 2020;160:1114–29. https://doi.org/10.1016/j.ijbiomac.2020.05.162.
Ding X, Suo Z, Sun Q, Gan R, Tang P, Hou Q, et al. Study of the interaction of broad-spectrum antimicrobial drug sitafloxacin with human serum albumin using spectroscopic methods, molecular docking, and molecular dynamics simulation. J Pharm Biomed Anal. 2018;160:397–403. https://doi.org/10.1016/j.jpba.2018.07.053.
Gan N, Sun Q, Zhang M, Tang P, Zhao L, Xie T, et al. Insights into the interaction of ulipristal acetate and human serum albumin using multi-spectroscopic methods, molecular docking, and dynamic simulation. J Biomol Struct Dyn. 2018B;37:2989–98. https://doi.org/10.1080/07391102.2018.1502686.
Marković OS, Cvijetić IN, Zlatović MV, Opsenica IM, Konstantinović JM, Jovanović NVT, et al. Human serum albumin binding of certain antimalarials. Spectrochim Acta A Mol Biomol Spectrosc. 2018;192:128–39. https://doi.org/10.1016/j.saa.2017.10.061.
Siddiqui MF, Khan MS, Husain FM, Bano B. Deciphering the binding of carbendazim (fungicide) with human serum albumin: a multi-spectroscopic and molecular modelling studies. J Biomol Struct Dyn. 2019;37:2230–41. https://doi.org/10.1080/07391102.2018.1481768.
Singh IR, Mitra S. Interaction of chlorpropamide with serum albumin: effect on advanced glycated end (AGE) product fluorescence. Spectrochim Acta A Mol Biomol Spectrosc. 2019;206:569–77. https://doi.org/10.1016/j.saa.2018.08.055.
Xie LX, Wu HL, Kang C, Xiang SX, Yin XL, Gu HW, et al. Quantitative investigation of the dynamic interaction of human serum albumin with procaine using a multi-way calibration method coupled with three-dimensional fluorescence spectroscopy. Anal Methods. 2015;7:6552–60. https://doi.org/10.1039/c5ay00790a.
Nasruddin AN, Feroz SR, Mukarram AK, Mohamad SB, Tayyab S. Fluorometric and molecular docking investigation on the binding characteristics of SB202190 to human serum albumin. J Lumin. 2016;174:77–84. https://doi.org/10.1016/j.jlumin.2016.02.004.
Cheng LY, Fang M, Bai AM, Ouyang Y, Hu YJ. Insights into the interaction of methotrexate and human serum albumin: a spectroscopic and molecular modeling approach. Luminescence. 2017;32:873–9. https://doi.org/10.1002/bio.3267.
Vaneková Z, Hubčík L, Toca-Herrera JL, Furtműller PG, Valentová J, Mučaji P, et al. Study of interactions between amlodipine and quercetin on human serum albumin: spectroscopic and modeling approaches. Molecules. 2019;24:487 https://doi.org/10.3390/molecules24030487.
Paul BK, Ray D, Guchhait N. Spectral deciphering of the interaction between an intramolecular hydrogen bonded ESIPT drug, 3, 5-dichlorosalicylic acid, and a model transport protein. Phys Chem Chem Phys. 2012;14:8892–902. https://doi.org/10.1039/c2cp23496c.
Yang H, Huang Y, Wu D, Yan J, He J, Li H. In vitro investigation of the interaction between the hepatitis C virus drug sofosbuvir and human serum albumin through 1H NMR, molecular docking, and spectroscopic analyses. N. J Chem. 2016;40:2530–40. https://doi.org/10.1039/C5NJ02003D.
Maurya N, Maurya JK, Singh UK, Dohare R, Zafaryab M, Moshahid Alam Rizvi M, et al. In vitro cytotoxicity and interaction of noscapine with human serum albumin: effect on structure and esterase activity of HSA. Mol Pharm. 2019;16:952–66. https://doi.org/10.1021/acs.molpharmaceut.8b00864.
Alsaif NA, Wani TA, Bakheit AH, Zargar S. Multi-spectroscopic investigation, molecular docking and molecular dynamic simulation of competitive interactions between flavonoids (quercetin and rutin) and sorafenib for binding to human serum albumin. Int J Biol Macromol. 2020;165:2451–61. https://doi.org/10.1016/j.ijbiomac.2020.10.098.
Gowda JI, Nandibewoor ST. Binding and conformational changes of human serum albumin upon interaction with 4-aminoantipyrine studied by spectroscopic methods and cyclic voltammetry. Spectrochim Acta A Mol Biomol Spectrosc. 2014;124:397–403. https://doi.org/10.1016/j.saa.2014.01.028.
Shahabadi N, Khorshidi A, Moghadam NH. Study on the interaction of the epilepsy drug, zonisamide with human serum albumin (HSA) by spectroscopic and molecular docking techniques. Acta A Mol Biomol Spectrosc. 2013;114:627–32. https://doi.org/10.1016/j.saa.2013.05.092.
Bijari N, Moradi S, Ghobadi S, Shahlaei M. Elucidating the interaction of letrozole with human serum albumin by combination of spectroscopic and molecular modeling techniques. Res Pharm Sci. 2018;13:304 https://doi.org/10.4103/1735-5362.235157.
Yang H, Zeng Q, He Z, Wu D, Li H. Interaction of novel Aurora kinase inhibitor MK-0457 with human serum albumin: Insights into the dynamic behavior, binding mechanism, conformation and esterase activity of human serum albumin. J Pharm Biomed Anal. 2020;178:112962 https://doi.org/10.1016/j.jpba.2019.112962.
Mansouri-Torshizi H, Zareian-Jahromi S, Abdi K, Saeidifar M. Nonionic but water soluble, [Glycine-Pd-Alanine] and [Glycine-Pd-Valine] complexes. Their synthesis, characterization, antitumor activities and rich DNA/HSA interaction studies. J Biomol Struct Dyn. 2018;37:3566–82. https://doi.org/10.1080/07391102.2018.1520647.
Schiff P, Fant J, Horwitz S. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–7. https://doi.org/10.1038/277665a0.
Lluch A, Álvarez I, Muñoz M, Seguí MA, Tusquets I, García-Estévez L. Treatment innovations for metastatic breast cancer: Nanoparticle albumin-bound (NAB) technology targeted to tumors. Crit Rev Oncol Hematol. 2014;89:62–72.
Wiedenmann N, Valdecanas D, Hunter N, Hyde S, Buchholz TA, Milas L, et al. 130-nm albumin-bound paclitaxel enhances tumor radiocurability and therapeutic gain. Clin Cancer Res. 2007;13:1868–74.
Authier N, Gillet J-P, Fialip J, Eschalier A, Coudore F. Description of a short-term Taxol®- induced nociceptive neuropathy in rats. Brain Res. 2000;887:239–49.
Cohen NC Ed. Guidebook on molecular modeling in drug design. Gulf Professional Publishing. Academic Press, NY, 1996.
Pagadala NS, Nataraj S, Syed K, Tuszynski J. Software for molecular docking: a review. Biophys Rev Lett. 2017;9:91–102.
Chaudhary KK, Mishra N. A review on molecular docking: novel tool for drug discovery. Databases. 2016;4:1029.
Kiraz S, İnci D, Aydın R, Vatan Ö, Zorlu Y, Cavaş T. Antiproliferative activity of copper (II) glutamine complexes with N, N-donor ligands: synthesis, characterization, potentiometric studies and DNA/BSA interactions. J Molr Struct. 2019;1194:245–55. https://doi.org/10.1016/j.molstruc.2019.05.086.
Liu K, Yan H, Chang G, Li Z, Niu M, Hong M, et al. complexes derived from hydrazone Schiff base: synthesis, crystal structure, in vitro cytotoxicity and DNA/BSA interactions. Inorg Chim Acta. 2017;464:137–46. https://doi.org/10.1016/j.ica.2017.05.017.
Manikanda Mathavan VM, Thangaraj M, WeyhermullerT, Parameswari RP, Punitha V, Murthy NN, et al. Novel mononuclear Cu (II) terpyridine complexes: impact of fused ring thiophene and thiazole head groups towards DNA/BSA interaction, cleavage and antiproliferative activity on HepG2 and triple negative CAL-51 cell line. Eur J Med Chem. 2017;135:434–46. https://doi.org/10.1016/j.ejmech.2017.04.030.
Alinaghi M, Karami K, Shahpiri A, Momtazi-borojeni AA, Abdollahi E, Lipkowski J. A Pd (II) complex derived from pyridine-2-carbaldehyde oxime ligand: synthesis, characterization, DNA and BSA interaction studies and in vitro anticancer activity. J Mol Struct. 2020;1219:128479.
Anjomshoa M, Fatemi SJ, Torkzadeh-Mahani M, Hadadzadeh H. DNA-and BSA-binding studies and anticancer activity against human breast cancer cells (MCF-7) of the zinc (II) complex coordinated by 5, 6-diphenyl-3-(2-pyridyl)-1, 2, 4-triazine. Spectrochim Acta A Mol Biomol Spectrosc. 2014;127:511–20.
Ayyannan G, Mohanraj M, Gopiraman M, Uthayamalar R, Raja G. New Palladium(II) complexes with ONO chelated hydrazone ligand: Synthesis, characterization, DNA/BSA interaction, antioxidant and cytotoxicity. Inorg Chim Acta. 2020;512:1198681 https://doi.org/10.1016/j.ica.2020.119868.
Joksimović N, Petronijević J, Janković N, Baskić D, Popović S, Todorović D, et al. Synthesis, characterization, anticancer evaluation and mechanisms of cytotoxic activity of novel 3-hydroxy-3-pyrrolin-2-ones bearing thenoyl fragment: DNA, BSA interactions and molecular docking study. Bioorg Chem. 2019;88:102954 https://doi.org/10.1016/j.bioorg.2019.102954.
Ishtikhar M, Rabbani G, Khan RH. Interaction of 5-fluoro-5-deoxyuridine with human serum albumin under physiological and non-physiological condition: a biophysical investigation. Colloid Surf B Biointerfaces. 2014;123:469–77. https://doi.org/10.1016/j.colsurfb.2014.09.044.
Bornmann L, Mutz R. Growth rates of modern science: a bibliometric analysis based on the number of publications and cited references. J Assoc Inf Sci Technol. 2015;66:2215–22.
Paál K, Müller J, Hegedûs L. High affinity binding of paclitaxel to human serum albumin. Eur J Biochem. 2001;268:2187–91.
Katayama NK, Nakajou Y, Ishima S, Ikuta J, Yokoe F, Yoshida A, et al. Nitrosylated human serum albumin (SNO-HSA) induces apoptosis in tumor cells. Nitric Oxide. 2010;22:259–65.
Otagiri M, Chuang VTG. Albumin in medicine. Springer, Singapore, 2016. https://doi.org/10.1007/978-981-10-2116-9.
Acknowledgements
The authors would like to thank the Foundation of Support to Sciences and Technology in Pernambuco [Fundação de Amparo à Ciência e Tecnologia de Pernambuco – FACEPE], Brazil, grant numbers APQ-0378-4.03/18 and APQ-0166-4.03/19) for the financial support. This study was partly funded by the Coordination for the Improvement of Higher Education Personnel, Brazil [Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)] - Finance Code 001. The authors are thankful to Doctor Larissa de P. Cavalcanti (laracvanti@gmail.com) from Universidade Federal Rural de Pernambuco, Unidade Acadêmica de Serra Talhada (UFRPE/UAST) for proof-reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ribeiro, A.G., Alves, J.E.F., Soares, J.C.S. et al. Albumin roles in developing anticancer compounds. Med Chem Res 30, 1469–1495 (2021). https://doi.org/10.1007/s00044-021-02748-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02748-z