Advertisement
Advertisement
July 2023
Sponsored by Inari Medical
Treating Venous Stent Occlusions With the Novel RevCore Thrombectomy System
An expert discussion and case reports on the first mechanical thrombectomy device to debulk in-stent thrombotic material and restore patency in failed or failing venous stents.
The number of venous stenting procedures has been increasing exponentially. In patients with postthrombotic syndrome (PTS) alone, these stents thrombose at a rate of up to 25% by 36 months.1 The lack of effective treatment options for failed venous stents has left a substantial unmet need to extract thrombotic material safely and restore patency.
In the acute stage, in-stent thrombosis (IST) is often treated with anticoagulation, thrombolysis, aspiration thrombectomy, or angioplasty. Subacute and chronic IST is treated with angioplasty, compression stockings, or the relining of stents, treatments that provide only temporary relief and do not effectively address or remove thrombotic material.2
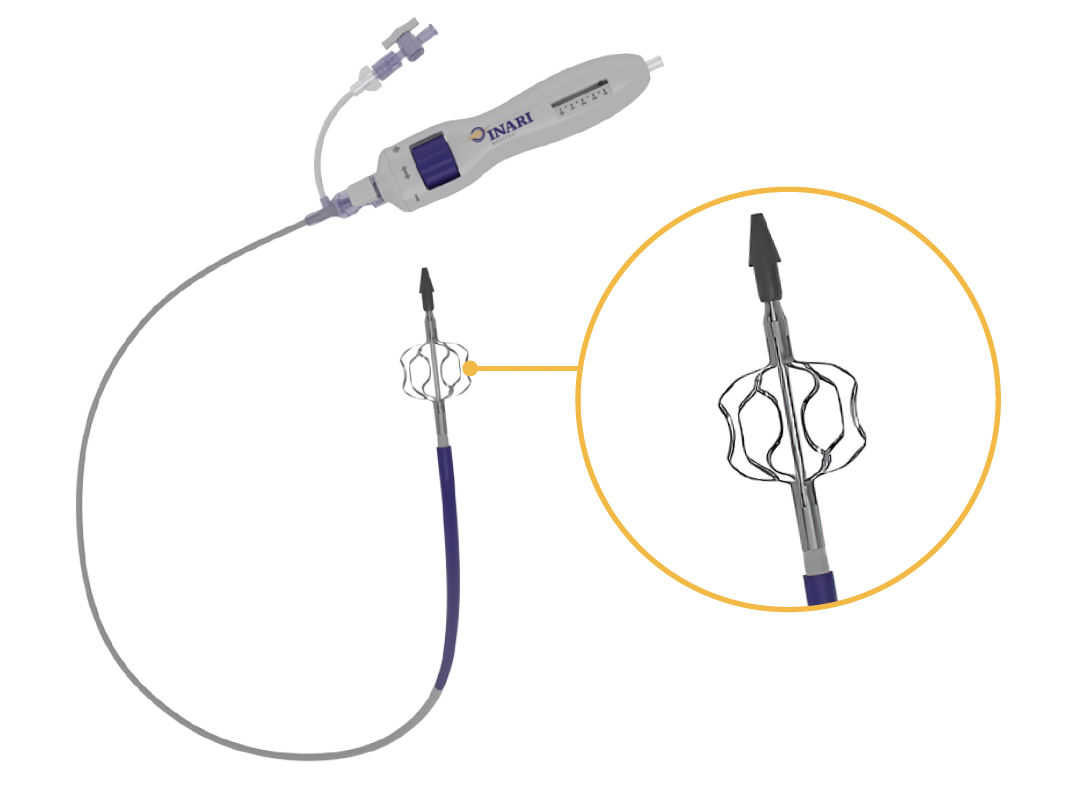
The RevCore Thrombectomy System (Inari Medical; Figure 1) is the first mechanical thrombectomy device to treat acute to chronic venous IST. It is a minimally invasive, over-the-wire device indicated for the treatment of thromboemboli in the peripheral vasculature, including implanted venous stents. RevCore consists of a catheter with a unique expandable element designed for safe and effective use in venous stents and a reinforced catheter shaft to allow retracting, advancing, torquing, and scrubbing movements for 360° vessel treatment. An external diameter-control knob enables sizing of the element to treat native vessels 6 mm or greater and stents ranging from 10 to 20 mm in diameter.
The introduction of this device marks a profound shift in the ability for physicians to improve outcomes for patients with IST. In this article, we interview vascular surgeon Dr. Steven Abramowitz about his experience treating patients with RevCore. Case reports from Dr. Abramowitz and Dr. Michael Siah follow.
1. Sebastian T, Spirk D, Engelberger RP, et al. Incidence of stent thrombosis after endovascular treatment of Iliofemoral or caval veins in patients with the postthrombotic syndrome. Thromb Haemost. 2019;119:2064-2073. doi: 10.1055/s-0039-1697955
2. Saleem T, Raju S. An overview of in-stent restenosis in iliofemoral venous stents. J Vasc Surg Venous Lymphat Disord. 2022;10:492-503.e2. doi: 10.1016/j.jvsv.2021.10.011
Q&A: RevCore in Daily Practice

Prior to RevCore, how did you treat IST?
One of the previous barriers to treating either failing or failed venous stents was that there were few effective tools to remove in-stent thrombotic material. For the most part, we were limited to balloon venoplasty, which could temporarily remodel material within the stent lumen. However, for many patients this was only marginally effective at making space to allow for a new stent to achieve maximal luminal expansion. This made the idea of relining an occluded venous stent unappealing, unless the patient had very advanced venous disease such as rapidly progressing C4, C5, or C6 disease. Ideally, we needed a device to significantly debulk the accumulated material and allow for appropriate treatment of the stent and inflow vessels.
Without RevCore, many of our patients with failing stents were relegated to lifelong compression and limited reintervention pathways with no end goal. This created a chronic and debilitating condition that could impact the patient’s quality of life.
Would you tell us about RevCore and how it has changed your treatment goals?
RevCore has the potential to make a monumental difference in treating patients with venous stent occlusion for primary assisted or secondary patency. There is currently no other tool like it for physicians to use. It is designed to work over the wire, with an expandable coring element that is externally controlled to treat diameters up to 20 mm. A reinforced catheter shaft makes it possible to perform a variety of movements to extract material from an occluded stent.
With RevCore, we’ve been able to provide a viable solution to maintain primary assisted patency or achieve secondary patency of venous stents. In the cases where we’ve used RevCore, the need for adjuvant interventions—such as stent relining—has been eliminated depending on the assessed mechanism of stent failure. It has given me the ability to better act on physical exam and noninvasive surveillance data indicating stent failure.
Let’s get into the specifics of a RevCore procedure. What is important to know about access?
One of the great things about the device is that it works through familiar access pathways, be it popliteal, femoral, or internal jugular (IJ) approaches. Many people won’t find this to be a departure from the access strategies they use for general treatment of venous disease. One consideration is that for those patients who have failed stents with significant inflow disease, there may be some challenges in bringing a larger-sized device through occluded or diseased femoropopliteal segments from a popliteal approach. However, the device handles very nicely once the segments are pretreated with balloon venoplasty.
Can you tell us more about the “revving” technique?
I always recommend you expose the element of the RevCore System first, and while in unexpanded (or collapsed) profile, bring the device back and forth through the stent. This ensures the device itself passes easily through the treatment segment and any inflow disease that may exist.
Next, I expand the RevCore element to a small diameter and “rev” the handle similar to rotating your wrist on a motorcycle throttle. Then, I pull back to the next segment and repeat. I’ll readvance in a back-and-forth motion, collapse and recapture the element, and remove the device from the body to assess the debulked material. This process is repeated with the element expanded at variable diameters until I’ve reached engagement with the stent itself.
One beneficial feature of the device is the haptic feedback—you are very much able to appreciate when the RevCore System is in contact with any exposed metal of the stent and redirect or contract the element as appropriate to avoid that contact and continue treatment. The process feels less like aggressive, large-volume debulking and rather like continual shaving of the material within the stent.
Is “revving” the primary movement you use with the device?
A great aspect of the device is that you’re able to move it at variable sizes of expansion, with or without “revving,” in a proximal and distal fashion. This adds a secondary method of material manipulation. When I think about the device, I think about three dimensions of movement: clockwise, counterclockwise, and back and forth. Together, these movements give you the ability to address the in-stent thrombotic material present.
Is there any possibility that RevCore could become entangled in the struts or otherwise cause any damage to a stent?
It’s a possibility, but with safe use and a staged approach to expansion, you have the potential to eliminate that possibility. You can always feel and visualize under fluoroscopy when the stent has been engaged by the device, and after a counter-rotation, you can feel when it disengages. Should you notice that there’s any stent deformation or contact with the stent, stop the “revving” action and dial down the element.
Are there any other procedural risks you consider, and how do you mitigate them?
Distal embolization is a known risk with any intervention associated with IST, and it was one of the things that was an early consideration in my RevCore cases since RevCore does not have a collection bag. Because this material may be chronic in nature, it would not respond to lytic-based pulmonary embolism interventional options. The use of a protective device to capture the mobilized material is certainly beneficial.
Are you able to forgo relining a stent after clearing thrombotic material with RevCore?
That is a clinical result we have been able to achieve and, when indicated based on inflow and outflow assessment, the treatment goal. That’s a huge strength here. With RevCore, we’ve been able to clear thrombotic material and delay relining at our practice. Oftentimes, our patients with nonthrombotic iliac vein lesions or PTS are not on long-term anticoagulation since there is no real level 1 guiding evidence for anticoagulation or antiplatelet management strategies for them. In our experience, RevCore allows us to be more strategic and carry out a successful thrombectomy procedure in conjunction with a potential change in medical management for those patients who had IST while off antiplatelet or anticoagulation agents. Primary assisted patency—or, in some cases, secondary patency—is therefore preserved and the need for restenting potentially delayed.
How important is intravascular ultrasound (IVUS) in these procedures?
I think IVUS is instrumental for a few reasons. First, it allows you to verify that the pathway you’re crossing is within the lumen of the stent. Second, it allows you to target your therapy to areas that may have residual disease that may not be appreciated on venography. Finally, IVUS gives me the ability to determine technical success more so than venography alone.
What is the learning curve for RevCore?
From a procedural standpoint, the learning curve shouldn’t be a barrier to early adoption. One can become very facile with the device after only a few procedures. The complexities of using RevCore to its full potential related to the larger problems of treating venous occlusive disease. You can’t use the device if you can’t cross the stent. For some people with limited experience in crossing chronically occluded venous stents, there’s going to be a learning curve to implement safe crossing techniques, particularly in making sure there is appropriate intraluminal crossing and that the procedures are well-planned and well-imaged in advance. Additionally, barriers to treating patients with poor inflow remain barriers to longer-term procedural success and stent patency.
Beyond patency, what are some of the markers you look for to determine procedural success?
The primary determinant of technical success when using RevCore is restoration of the lumen of the stent. I think some secondary markers of technical success would be reduction of flow into formed collaterals, improved contrast “washout” in the affected extremity, and, potentially, the ability to prevent stent relining.
How do you think RevCore will change the treatment pathway and the long-term follow-up for patients with venous stents?
In my mind, what RevCore does is a game-changer in terms of the treatment algorithm for these patients.
Before RevCore, I and most of my colleagues made clinical decisions around venous stent occlusion that were predicated primarily on disease-state severity and a risk-benefit assessment regarding reocclusion risk. Then, when we reached a terminus of the ability to intervene, the patients were relegated to medical therapy. RevCore essentially changes that point of terminus. We no longer have to rely on conservative medical therapy or relining for a failed stent. We now have another option, and I think that makes a very big difference in the clinical approach and algorithm for managing these patients.
What would you like to highlight for other physicians looking to add RevCore to their toolkit?
Physicians will need to consider the potential impact on how we follow and recommend reintervention for these patients. That includes how we can recover patients who are under the impression that there’s nothing more to be done, which is a huge barrier. There are plenty of patients with occluded stents in wound care centers right now who have been told there are no more options.
I would also emphasize the potential reduced need to reline stents with this new device. The key feature about RevCore is that it changes the likelihood of successful reintervention for primary assisted and secondary patency. I’m not saying that we’re never going to need to reline a stent again, but the likelihood of a relined stent remaining patent is going to be higher because you’re more likely to see appropriate luminal gain beyond what you would have previously anticipated. Even in cases where you may have to reline the stent, I am optimistic that RevCore would provide a favorable debulked segment to make the reintervention a success. A few months out from the first RevCore procedures, we’ve actually seen patient progression in those treated without relining, and for me that’s a very optimistic sign!
Patency and Brisk Flow Restored in Patient With a 15-Year History of Symptomatic Left Iliac Venous Stent Occlusion
By Steven Abramowitz, MD
PATIENT PRESENTATION
A woman in her early 60s with a history of venous thromboembolism had an inferior vena cava (IVC) filter placed in 2005. Two years later, she underwent thrombolysis and placement of a Wallstent (Boston Scientific Corporation) in the left common iliac vein (CIV) to treat partial IVC and IVC filter thrombosis. The stent occluded within 1 year, and the patient has had chronic limb swelling managed by compression ever since. Given worsening CEAP (clinical, etiologic, anatomic, and pathophysiologic) C4 changes, a decision was made to treat the IST with the RevCore System. The IVC filter was removed in preparation of the procedure.
PROCEDURAL OVERVIEW
Ultrasound guidance was used to access the right and left popliteal and right IJ veins. Micropuncture catheters were placed and then exchanged for 9-F sheaths. Ascending venography confirmed the left iliac stent occlusion (Figure 1A). The occlusion was crossed using a supporting 55-cm, 5-F sheath, a stiff angled Glidewire (Terumo Interventional Systems), and a Glidecath catheter (Terumo Interventional Systems). The sheath was removed, and the Glidewire was snared from the right IJ access. IVUS confirmed wire placement within the stent lumen (Figure 1B). The Glidecath and Glidewire were replaced with a 135-cm Quick-Cross support catheter (Philips) and Lunderquist wire (Cook Medical), respectively.
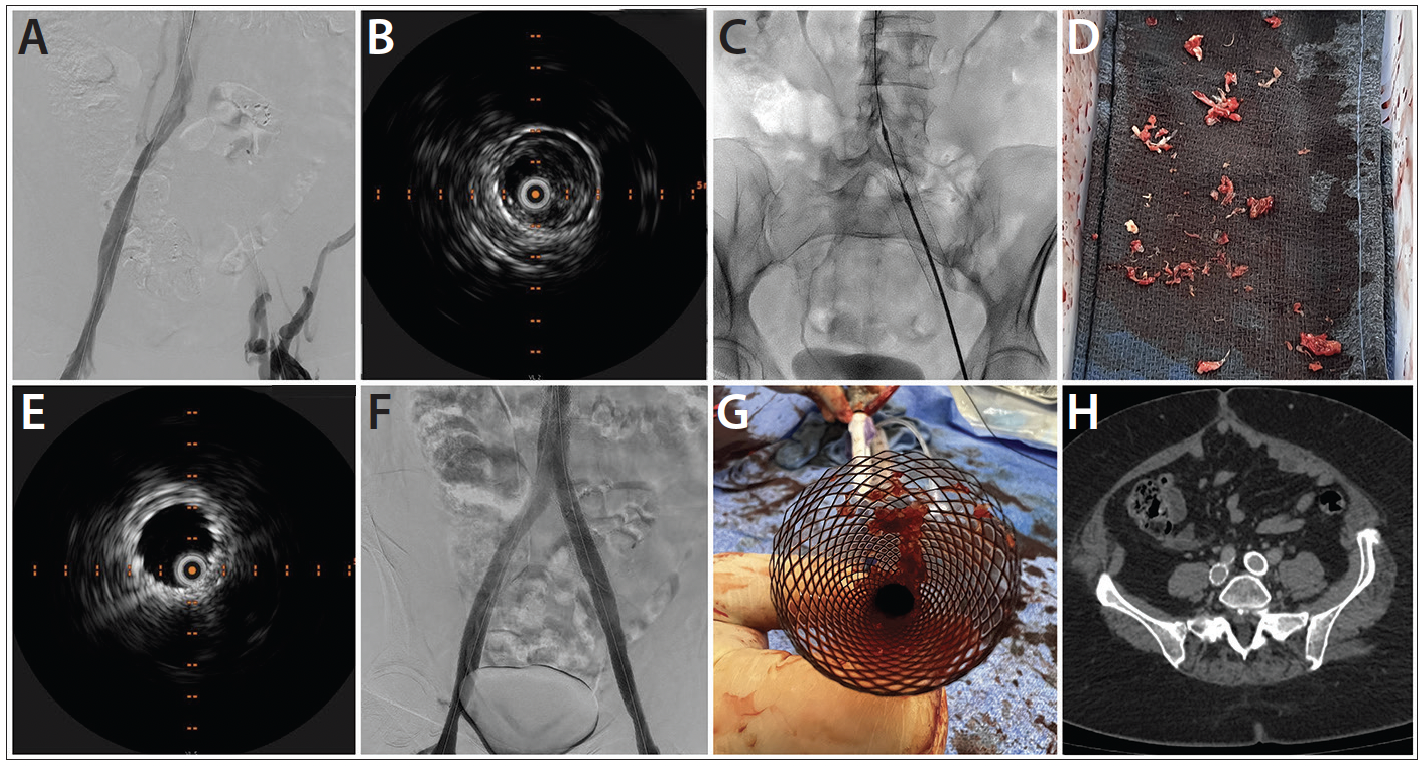
Figure 1. Preprocedural venogram of occluded left iliac vein stent (A). Preprocedural IVUS image confirms wire position within the stent lumen (B). RevCore coring element deployed (C). Extracted thrombotic material (D). Postprocedural IVUS confirms clearance of thrombotic material (E). Final venogram (F). Thrombus trapped in the Protrieve funnel (G). CT scan at 30-day follow-up (H).
After dilation, a Protrieve sheath (Inari Medical) was deployed in the infrarenal IVC. A 16-F ClotTriever sheath (Inari Medical) was advanced into the left popliteal vein in exchange for the 9-F sheath, and the left iliofemoral vessels were dilated. RevCore was advanced into the IVC in its unexpanded state, and the element retracted and advanced through the stent without resistance. The element was expanded to the first half-tick mark (Figure 1C), and the device rotated first counterclockwise, then clockwise, within the stent. RevCore was withdrawn and cleaned, and the process repeated with incremental expansion of the element over multiple passes. The Protrieve sheath was then aspirated, followed by aspiration within the stent using a Triever16 aspiration catheter (Inari Medical). Blood was returned using the FlowSaver Blood Return System (Inari Medical). RevCore was reinserted for another pass, and IVUS confirmed removal of 95% of thrombotic material from within the stent (Figure 1D). The IVC lesion was predilated, and a 24- X 70-mm Wallstent was deployed and postdilated. Abutting 14- X 140-mm and 14- X 80-mm Abre stents (Medtronic) were placed in the left and right iliac veins after predilation. IVUS confirmed successful stent deployment and no stent-stent gap in the left iliofemoral segment (Figure 1E). Venography showed patent and brisk flow (Figure 1F).
All devices were removed, and thrombotic material was seen in the Protrieve funnel (Figure 1G). Compression wraps were placed on both legs. Case time was approximately 112 minutes.
CONCLUSION
At 30-day follow-up, the patient’s venous claudication symptoms had resolved, and swelling had almost completely resolved. Stent patency was maintained per CT scan (Figure 1H). Previously at risk for severe venous disease, the patient’s Villalta score reduced to 5—a substantial improvement from the preoperative score of 14.
RevCore Treatment Restores Patency to Completely Occluded Left EIV and CIV Stents 8 Months After Iliocaval Reconstruction

PATIENT PRESENTATION
A man in his early 50s with a history of bilateral leg swelling and a healed left ankle ulceration underwent iliocaval reconstruction for caval atresia using three Wallstents and three Abre stents (Figure 1A). The patient was intermittently compliant with his antiplatelet and anticoagulation regimen and was seen in clinic 8 months later with a 1-month history of increased debilitating leg swelling. A venous duplex ultrasound revealed occluded left external iliac (EIV) and CIV stents (Figure 1B). The patient was scheduled for in-stent mechanical thrombectomy with the RevCore System.
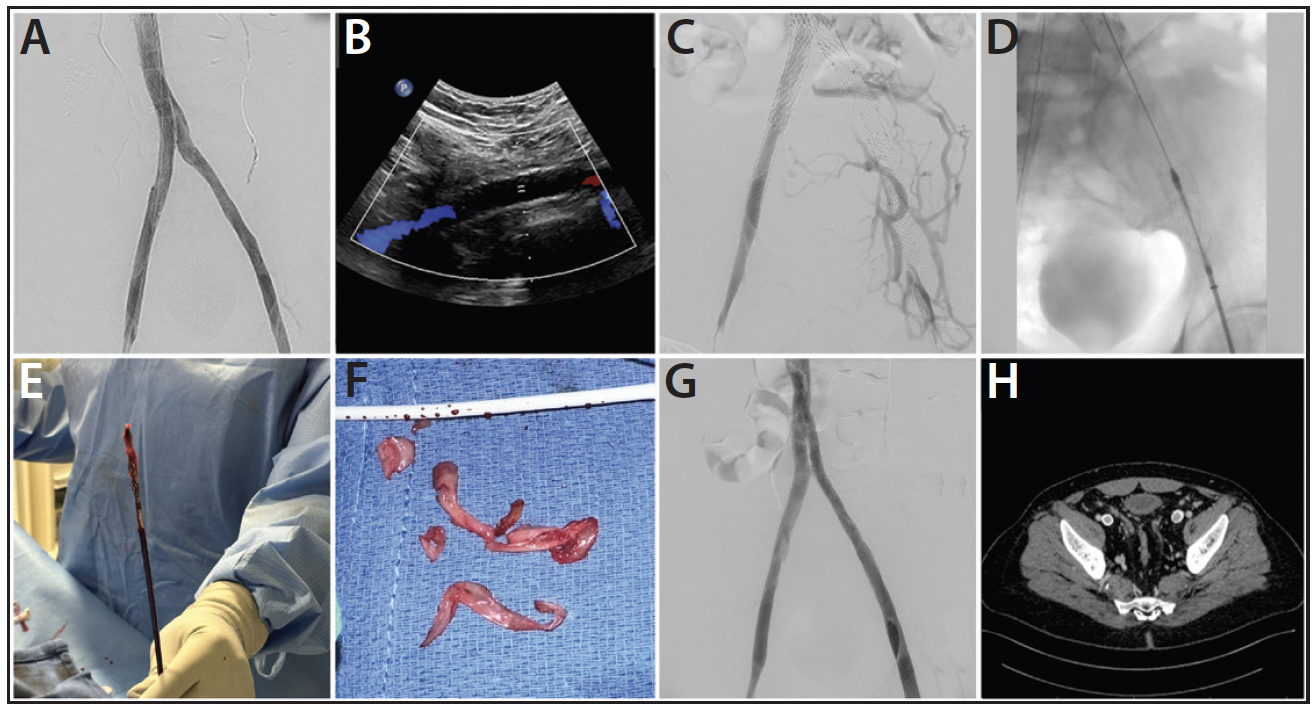
Figure 1. Iliocaval reconstruction completion venogram taken (A). Duplex ultrasound revealing an occluded left EIV stent (B). Initial venogram of present complaint (C). RevCore System positioned in the left EIV stent (D). Extracted thrombotic material (E, F). Completion venogram following thrombectomy (G). CT venogram confirming stent patency at 1-month follow-up (H).
PROCEDURAL OVERVIEW
The patient was positioned supine. The right IJ and bilateral groins and popliteal areas were prepped, and 9-F sheaths were placed in the right IJ and right common femoral vein (CFV). Venography confirmed completely occluded left EIV and CIV stents (Figure 1C). A short 16-F sheath was introduced through the left CFV access, and the left-sided stent occlusions were crossed using a Glidewire and Glidecath catheter. The Glidewire was snared from the right IJ access. IVUS was then performed along the length of both wires to look for stent damage and confirm that the wires were in the true lumen.
Serial dilatation to 20 F was performed on the right IJ access. A Protrieve sheath was introduced and advanced to the intrahepatic IVC where the Protrieve funnel was deployed, achieving full wall apposition as confirmed by IVUS. After predilatation, the RevCore was introduced through the Protrieve sheath and advanced to the left EIV and CIV stents. The RevCore element was unsheathed and remained unexpanded to perform an initial safety pass through the occluded stents. The element was then expanded until resistance was met. Full clockwise and counterclockwise rotations were performed until the element spun freely; it was then retracted by 5 to 10 mm and the process repeated until the pass was complete. Three RevCore passes were performed from the right IJ access, followed by five passes from the left CFV access (Figure 1D), removing substantial collagenous thrombus from the stents (Figure 1E and 1F). Aspiration thrombectomy was performed through the Protrieve sheath with a Triever16 catheter, and filtered blood was returned to the patient through the FlowSaver System. IVUS confirmed that very little material remained within the lumen of the stent. Following this, venoplasty was performed using a 14- X 60-mm Atlas ultra-noncompliant PTA balloon (BD Interventional). Final venography confirmed full patency and brisk flow through the previously occluded stents (Figure 1G).
All wires and catheters were removed. Hemostasis was achieved with manual pressure. Total procedure time was ~150 minutes; total device time was ~45 minutes. No further stenting or relining was required. Estimated blood loss was ≤ 100 mL. The patient was discharged the next day.
CONCLUSION
The patient’s pain resolved postprocedure, and the swelling improved greatly. At 1-month follow-up, the swelling was completely resolved. CT venography performed at follow-up revealed widely patent bilateral iliac vein stents (Figure 1H).
Advertisement
Advertisement